Interleukin-26 Differentially Modulates Human Macrophage Inflammatory Response to Distinct Mycobacterium tuberculosis Whole Cell Lysates
DOI:
https://doi.org/10.14740/cii507Keywords:
Mycobacterium tuberculosis, Macrophages, IL-26Abstract
Background: Interleukin-26 (IL-26) is an antimicrobial peptide that may contribute to the elimination of intracellular Mycobacterium tuberculosis (Mtb). The aim of the study was to investigate how IL-26 affected the response of human macrophages to Mtb whole cell lysates from different lineages.
Methods: Human macrophages were treated with IL-26 (monomer or dimer) before stimulation with Mtb lysates from Indo-Oceanic, HN878, East-African-Indian, and CDC1551 strains.
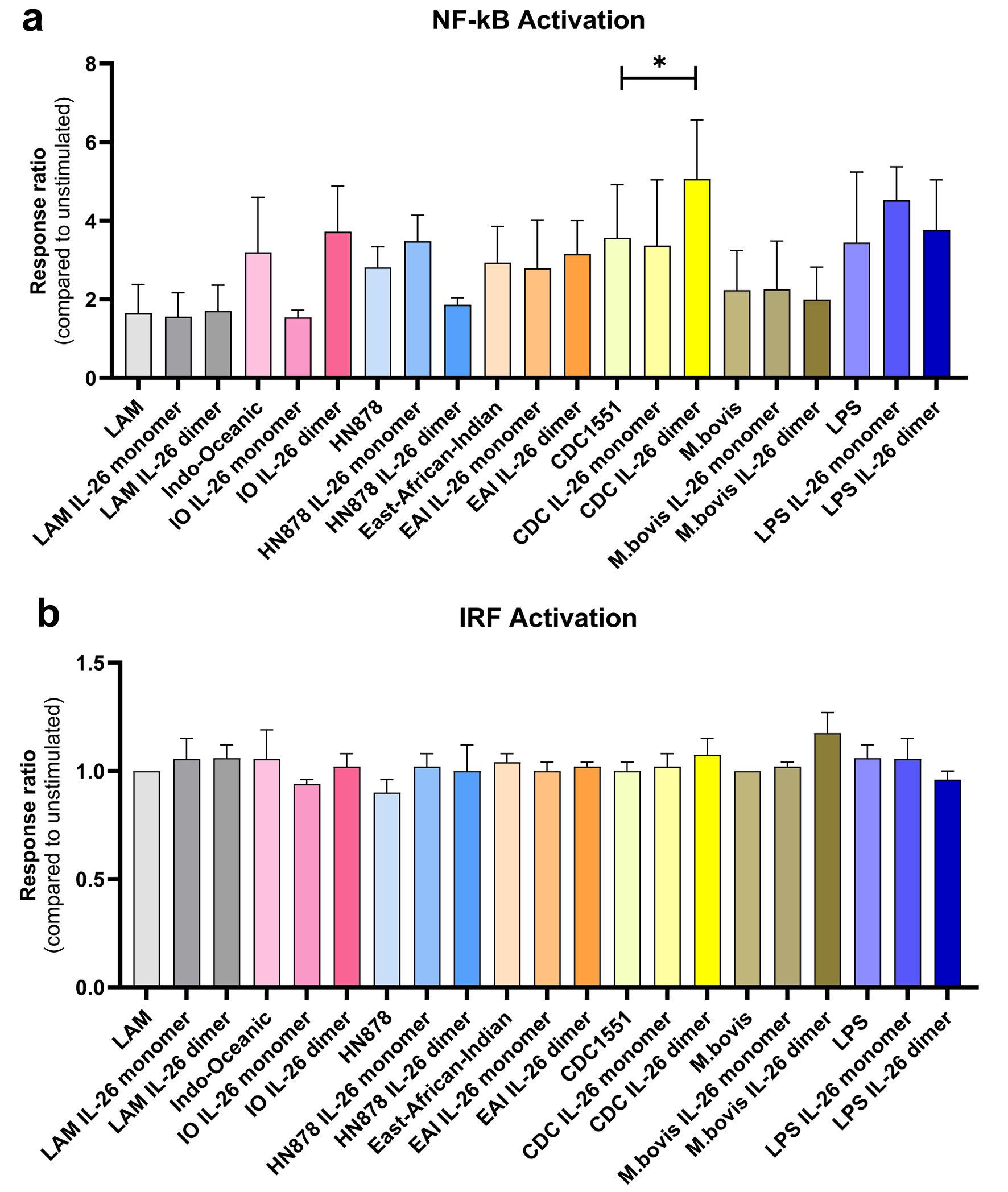
Results: Nuclear factor kappa B (NF-κB) activation was similar in untreated cells after stimulation with all lysates, but was diminished in IL-26 monomer-treated macrophages in response to Indo-Oceanic lysates. Macrophage exposure to dimeric IL-26 led to a higher NF-κB activation in response to CDC1551 lysates, but lower to HN878 lysates. No changes were observed in interferon regulatory factor (IRF) activation. Overall, IL-26 modulates NF-κB activation in a strain and confirmation-dependent manner. This modulation influences the macrophage inflammatory response to Mtb. Our results suggest that IL-26 may promote M1 polarization and enhance anti-Mtb immunity through the NF-κB pathway. This is particularly true in response to high-cytokine-inducing strains like CDC1551.
Conclusion: More studies are needed to clarify IL-26’s dual role in inflammation and immune regulation, and to explore its therapeutic potential in tuberculosis (TB) treatment and vaccine strategies.
Published
Issue
Section
License
Copyright (c) 2025 The authors

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






