| Clinical Infection and Immunity, ISSN 2371-4972 print, 2371-4980 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Clin Infect Immun and Elmer Press Inc |
| Journal website https://cii.elmerpub.com |
Review
Volume 000, Number 000, September 2025, pages 000-000
Acute Spinal Infections: A Modern Narrative Review With Evidence Through 2024
Eti Muharremia, Francis Demirajb, f, Denis Babicib, Ayleen Shabanc, Tugce Kutukd, Sujai Nathe
aDepartment of Neurology, Cooper University. Camden, NJ, USA
bDepartment of Neurology, FAU Charles E. Schmidt College of Medicine, Boca Raton, FL, USA
cKiran C. Patel College of Osteopathic Medicine, Nova Southeastern University, Davie, FL, USA
dDepartment of Radiation Oncology, The Ohio State University Wexner Medical Center, Columbus, OH, USA
eMarcus Neuroscience Institute, Baptist Health, Boca Raton, FL, USA
fCorresponding Author: Francis Demiraj, Department of Neurology, FAU Charles E. Schmidt College of Medicine, Boca Raton, FL, USA
Manuscript submitted June 11, 2025, accepted August 7, 2025, published online September 12, 2025
Short title: A Review of Acute Spinal Infections
doi: https://doi.org/10.14740/cii509
- Abstract
- Introduction
- Selection Criteria
- Classification and Pathophysiology
- Clinical Presentation and Diagnosis
- Management
- Limitations
- Conclusions
- References
| Abstract | ▴Top |
Spinal infections are serious conditions affecting either the spinal canal or surrounding structures (e.g., vertebrae, the intervertebral discs or paraspinal tissues) and are caused by different organisms including bacteria and fungi. The most common mechanism is either hematogenous spread or after surgical procedures. It is important to make a prompt diagnosis based on clinical manifestations, as well as supporting laboratory and imaging testing. Depending on the localization and the severity of the spinal infections, management is either conservative, with antimicrobials in neurologically stable patients with no signs of spinal instability, or additional surgical treatment when there is spinal cord compression or instability. Early diagnosis and treatment remain crucial to avoid serious complications and poor outcomes.
Keywords: Spinal infections; Instability; Surgical treatment
| Introduction | ▴Top |
Spinal infections (SIs) are a term used to describe a spectrum of infectious diseases that affect either the vertebrae, the intervertebral discs, the spinal canal and the space surrounding it, or adjacent paraspinal soft tissues [1]. These are classified respectively as vertebral osteomyelitis, discitis, spondylodiscitis, spinal epidural abscess (SEA), and paraspinal muscle abscesses [2]. However, infection usually spreads and involves different compartments of the spinal column and is rarely confined to only one of the mentioned anatomical structures [3].
They are either caused by bacteria or fungi, with Staphylococcus aureus (S. aureus) being the most common organism identified. Other frequent bacterial pathogens are coagulase-negative Staphylococci, Streptococci, and Escherichia coli [4]. Most common fungal agents causing SI are Aspergillus spp., Candida spp., and Cryptococcus neoformans, but they are not so easily identified and occur less frequently [4]. No organism can be identified in one-third of patients.
SIs most commonly affect the lumbar spine, followed by thoracic, cervical, and to a lesser extent sacral spine [4]. Direct open spinal trauma or hematogenous dissemination from a distant site, as well as contiguous spread occurring up to 3 months after surgical procedures are the described responsible mechanisms [5]. SIs are predominantly seen in males, with male/female ratio ranging between 2:1 and 5:1 [6, 7]. Although SIs can occur at any age, vertebral spondylodiscitis predominantly affects adults, with a higher prevalence among individuals over 50 years old, likely due to increased rates of immunosuppression, immunosuppressive therapy, and surgical implant use in this group. Among younger age groups, intravenous drug abusers have a high risk of developing SIs [8].
The incidence of SI has increased in recent years, probably due to the aging population and increased number of patients with chronic diseases, growing number of intravenous drug abusers, increased spinal trauma and surgery, as well as increased sensitivity of imaging techniques used currently [9]. An epidemiological study in the United States confirmed increased incidence of vertebral osteomyelitis from 2.9/100,000 in 1998, to 5.4/100,000 in 2013 [10]. This narrative review aims to present current knowledge spanning the classification, pathophysiological processes, clinical manifestations, and therapeutic approaches associated with SIs.
| Selection Criteria | ▴Top |
We included English-language, full-text articles addressing acute SIs in humans, encompassing original research (cohort studies, case series), clinical guidelines, and narrative or systematic reviews. We excluded animal or in vitro studies and records without accessible full text.
| Classification and Pathophysiology | ▴Top |
SIs are named and classified after the respective anatomical structure affected within the spine. In clinical practice, the infection is usually not confined to a single anatomic structure. For example, the involvement of both the disc and vertebral bodies is named spondylodiscitis, with imaging features displayed in Figure 1 [11]. As shown in Figure 2 [12], magnetic resonance imaging (MRI) findings in discitis typically demonstrate destruction of opposed vertebral endplates and fluid collection within the disc space. Given the vascular structure of the spine, a single vascular pedicle typically branches to supply two neighboring vertebral end plates. Consequently, in most instances, an infection affects two adjacent vertebral bodies along with their intermediate disc. Characteristic MRI features of spondylitis, including hypointense signal on T1-weighted images and hypointense edema on T2-weighted images, are illustrated in Figure 3 [13]. The initial site of infection is usually the vertebral end plates, and over time, the infection may spread to the adjacent disc or vertebral body. Additionally, the slow blood flow in these vessels, the absence of valves, and the complexity of the arterial or venous supply increase the vulnerability of the vertebral column to infections in individuals with bacteremia.
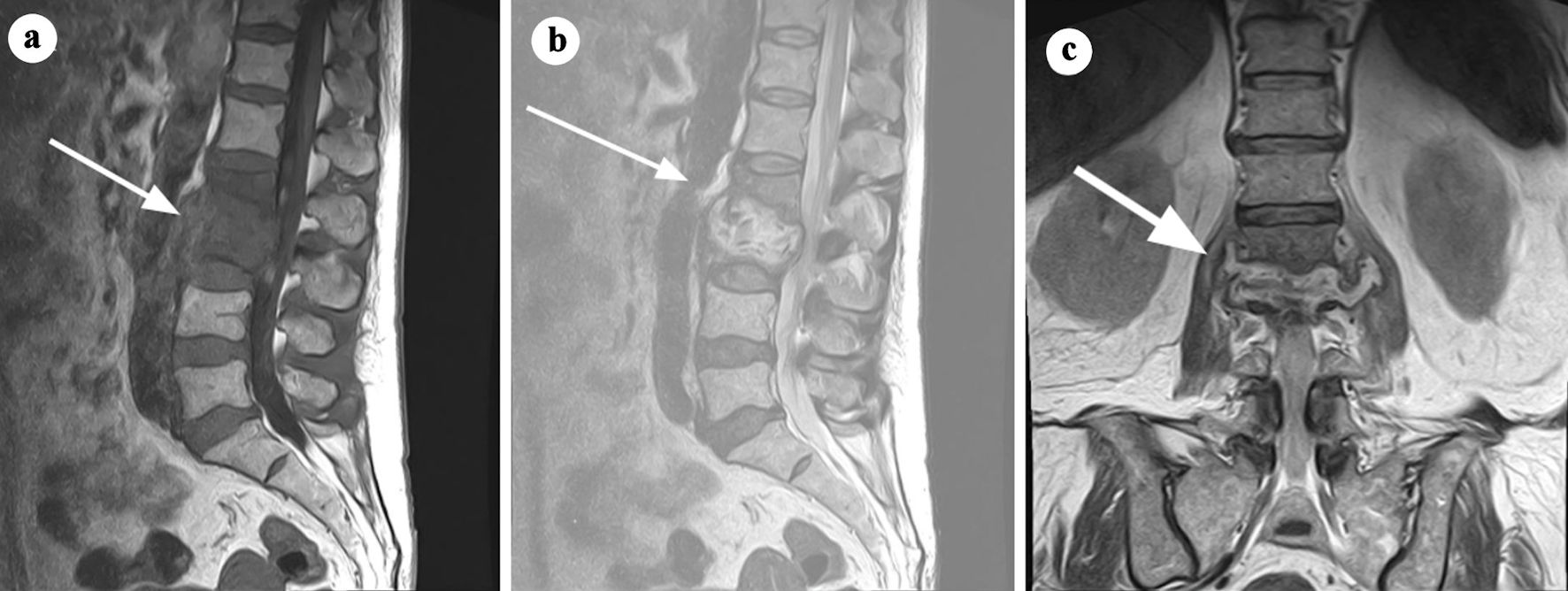 Click for large image | Figure 1. MRI findings consistent with spondylodiscitis at the L2-L3 level. (a) T1-weighted sagittal MRI showing hypointense signal within the L2-L3 intervertebral disc and adjacent vertebral endplates, indicative of inflammation and structural damage (arrow). (b) T2-weighted sagittal MRI demonstrating a hyperintense signal in the L2-L3 intervertebral disc, reflecting edema and fluid collection (arrow). (c) Coronal short tau inversion recovery (STIR) MRI highlighting an elevated signal infiltrating the L3 vertebral body, superior and inferior endplates of L2, and the paravertebral space, involving the psoas muscles and posterior wall (arrow). These findings result in neuroforaminal stenosis with contact to the emerging nerve roots at L2-L3, suggestive of an infectious process with possible infiltrative characteristics. Adapted from Gonzalez Herrera et al [11] (rID: 188070). MRI: magnetic resonance imaging. |
 Click for large image | Figure 2. Sagittal MRI images of discitis at the T8/T9 level. (a) T1-weighted image showing destruction of the opposed endplates and loss of vertebral body height at T8/T9 (arrow). (b) T2-weighted image demonstrating fluid collection within the disc space and extensive phlegmon in the surrounding paravertebral soft tissues (arrow). (c) Post-contrast T1-weighted imaging with fat saturation demonstrates enhancement of the phlegmon involving the paravertebral and epidural spaces, resulting in focal spinal canal narrowing (arrow). No evidence of epidural abscess is observed. Adapted from Di Muzio et al [12]. MRI: magnetic resonance imaging. |
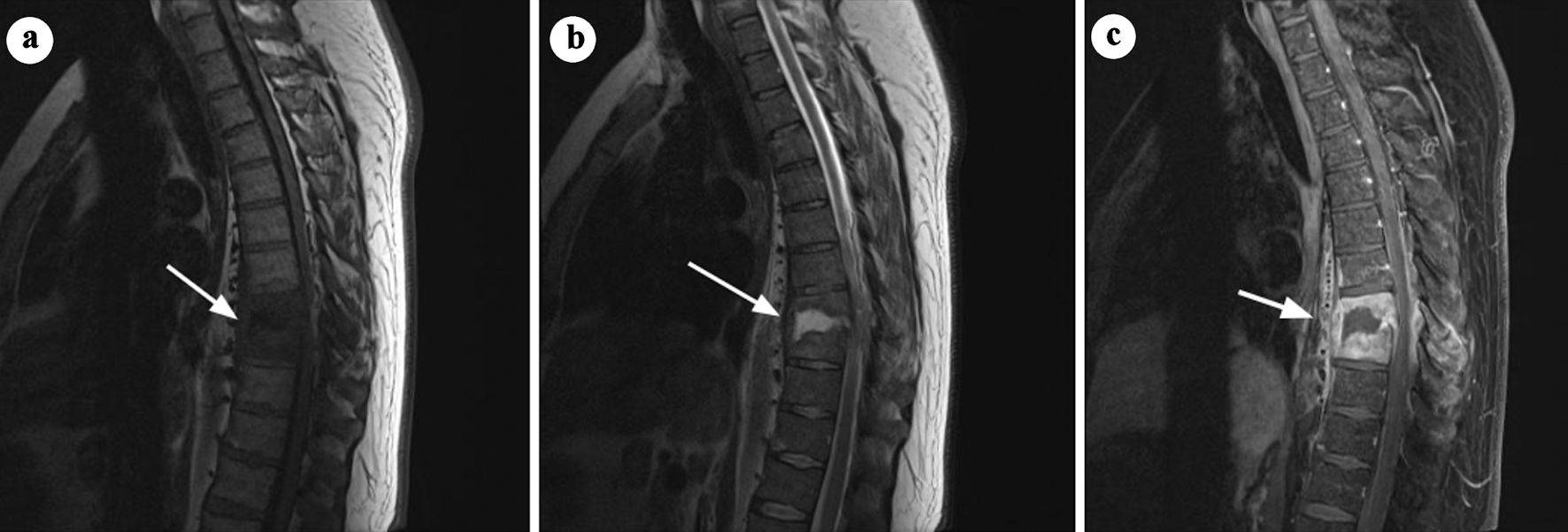
 Click for large image | Figure 3. Sagittal and axial MRI images demonstrating features of spondylitis at the L3-L4 level. (a) T1-weighted sagittal MRI showing hypointense signal in the L3-L4 vertebral endplates and adjacent vertebral bodies, consistent with inflammation (arrow). (b) T2-weighted sagittal MRI illustrating hyperintense signal in the affected region, indicative of edema (arrow). (c) Axial and sagittal short tau inversion recovery (STIR) images with contrast enhancement highlighting abnormal enhancement in the L3-L4 region. Small paraspinal abscesses are also identified, confirming active infection. Adapted from Aguiar et al [13] (rID: 67347). MRI: magnetic resonance imaging. |
Infectious agents reach the spine via three routes: 1) direct external inoculation after trauma or surgery; 2) hematogenous spread from a distant primary site; 3) contiguous spread from an adjacent tissue [14].
Direct inoculation consists in the presentation of the causative agents in the corresponding tissues because of the barrier damage, either after trauma or surgery. Hematogenous dissemination involves the migration of microorganisms from distant primary sites - such as the genitourinary, gastrointestinal, or respiratory tracts, oral cavity, heart (in cases of endocarditis), or soft tissues - to the spine via arterial circulation or retrograde spread through the valveless Batson venous plexus. Notably, in nearly 50% of cases, the original source of infection remains unidentified [15]. Any event causing microorganisms to enter the bloodstream, including surgical procedures or even minor activities such as tooth brushing or venipuncture, can theoretically lead to hematogenous spondylodiscitis [13]. Urinary tract infections, particularly those related to genitourinary interventions, are the most common source of transient bacteremia and subsequent SIs [1, 11, 12]. On rare occasions, infection may spread directly from neighboring structures such as the aorta, esophagus, or bowel [16, 17]. Because of the vascular anatomy of the spine, two consecutive vertebral end plates are supplied by the same vessels for a pedicle, therefore, in most cases infection involves two adjacent vertebral bodies and the respective intervertebral disc [11, 12, 14].
| Clinical Presentation and Diagnosis | ▴Top |
Clinical presentation and diagnosis of SIs are critical for timely and accurate management. SI often manifests with nonspecific symptoms, making it challenging to diagnose. The typical clinical presentation includes pain, fever and paresis. Among those, pain is the most common of the presenting clinical signs [18], and it is usually more pronounced at night and can even be severe enough to awaken the patient. It may be in the neck or back, depending on the level of infection and may radiate to the lower limbs, abdomen or groin. The pain does not have any specific qualities to differentiate it from the more common mechanical back pain, but the coexistence of fever should raise the clinical suspicion. Fever is another common clinical sign among SIs, but it is only present in approximately 50% of the patients with bacterial SIs [18]. This percentage is further reduced in patients with fungal, mycobacterial, or brucellar infections, as these individuals are often afebrile. The same study revealed that a neurological deficit such as paresthesia, radiculopathy or limb weakness/paralysis and loss of sphincter function, was present in 47% of the patients [18]. Due to the low specificity of signs and symptoms, diagnosis is delayed. Overall, pain remains the most common and usually the only clinical sign on presentation, therefore a high index of clinical suspicion is needed when assessing population at risk for SIs and should be followed by imaging and laboratory findings to make a diagnosis.
Routine laboratory markers are important in evaluating a patient with suspected SI. Evaluating the white blood cell (WBC) count alongside inflammatory markers such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) is a crucial part of the initial diagnostic assessment. WBC is the least sensitive among these tests, and a normal WBC count does not rule out a diagnosis of SI. CRP and ESR on the other hand are more sensitive markers, with CRP having the highest sensitivity (98%), while elevated ESR is observed in approximately 75% of cases [19].
Despite its high incidence, the sole elevation of CRP can be misleading and is not sufficient to suggest a diagnosis of SI by itself. In patients presenting with back pain and fever, elevated CRP levels alongside an increased ESR or WBC count are suggestive of a SI. CRP also serves as a valuable marker for treatment response, as it tends to normalize quickly following effective therapy, unlike ESR, which may remain elevated in up to 50% of cases despite clinical improvement [20]. Procalcitonin (PCT) serves as a valuable biomarker for differentiating bacterial infections from non-bacterial causes and can assist in guiding the initiation of empiric antibiotic therapy. Blood cultures, preferably taken during a fever spike, are part of the workup in patients with suspected SI to identify the microorganism responsible, and they should be collected before starting empiric antibiotic therapy [21]. The offending microorganism is identified in up to 59% of blood cultures [22], so sometimes a superior diagnostic approach is needed, like computed tomography (CT)-guided biopsy of infected tissue [23]. In patients from endemic regions or those with a high likelihood of disease, additional testing - such as Brucella serology, Mycobacterium tuberculosis screening (purified protein derivative (PPD) or interferon-γ release assay), and fungal assays - should be performed alongside the standard diagnostic workup [19].
Imaging studies are necessary to reveal the location and extent of the lesion, and it usually includes radiography and MRI of the spine [24]. Full spinal imaging is essential to evaluate the extent of infection and to rule out adjacent or skip lesions. Plain radiographs are frequently the initial imaging study due to their accessibility and low cost; however, abnormalities typically appear late in the disease process, and the overall sensitivity remains low. Radiographic visualization of osseous demineralization requires 30-40% loss of the osseous matrix, which may occur up to 2 weeks after onset of clinical symptoms [25]. CT is not considered to be of much use for diagnosis of SI, however, it is often utilized to obtain samples of infected specimens, for both osseous and non-osseous tissue, as mentioned above. MRI is the modality of choice for diagnosis of SIs, with reported sensitivity of 96% and specificity of 93% [26, 27]. Bone marrow edema manifests as hypo-intensity on T1-weighted imaging (T1WI) and hyperintensity on T2-weighted imaging (T2WI) within the intervertebral discs, vertebral bodies or epidural space [11, 28]. Radiographic findings of a SEA are illustrated in Figure 4 [29]. Loss of endplate definition is also a characteristic finding. Contrast imaging is recommended if there are no contraindications to gadolinium, to increase the sensitivity. Contrast enhancement of the vertebral body and disc are appreciated on T1W images. Although MRI is considered the imaging modality of choice, some patients may have contraindications requiring alternative imaging approaches. In such cases, radionuclide imaging (RI), particularly gallium-67 single-photon emission computed tomography (SPECT), is preferred and has demonstrated strong efficacy as an alternative to MRI [30]. Bone scintigraphy using technetium-99m or indium-111 tends to have lower sensitivity. While 18F-fluorodeoxyglucose (FDG) PET is a viable alternative, it is important to recognize that FDG uptake occurs not only at sites of infection but also in areas of inflammation, including those associated with autoimmune and granulomatous conditions [31]. Lack of specificity is a limiting factor for PET, as radionuclide uptake can occur in a variety of inflammatory and neoplastic processes, hence clinicians must consider PET findings in the context of the clinical and previous imaging findings [32].
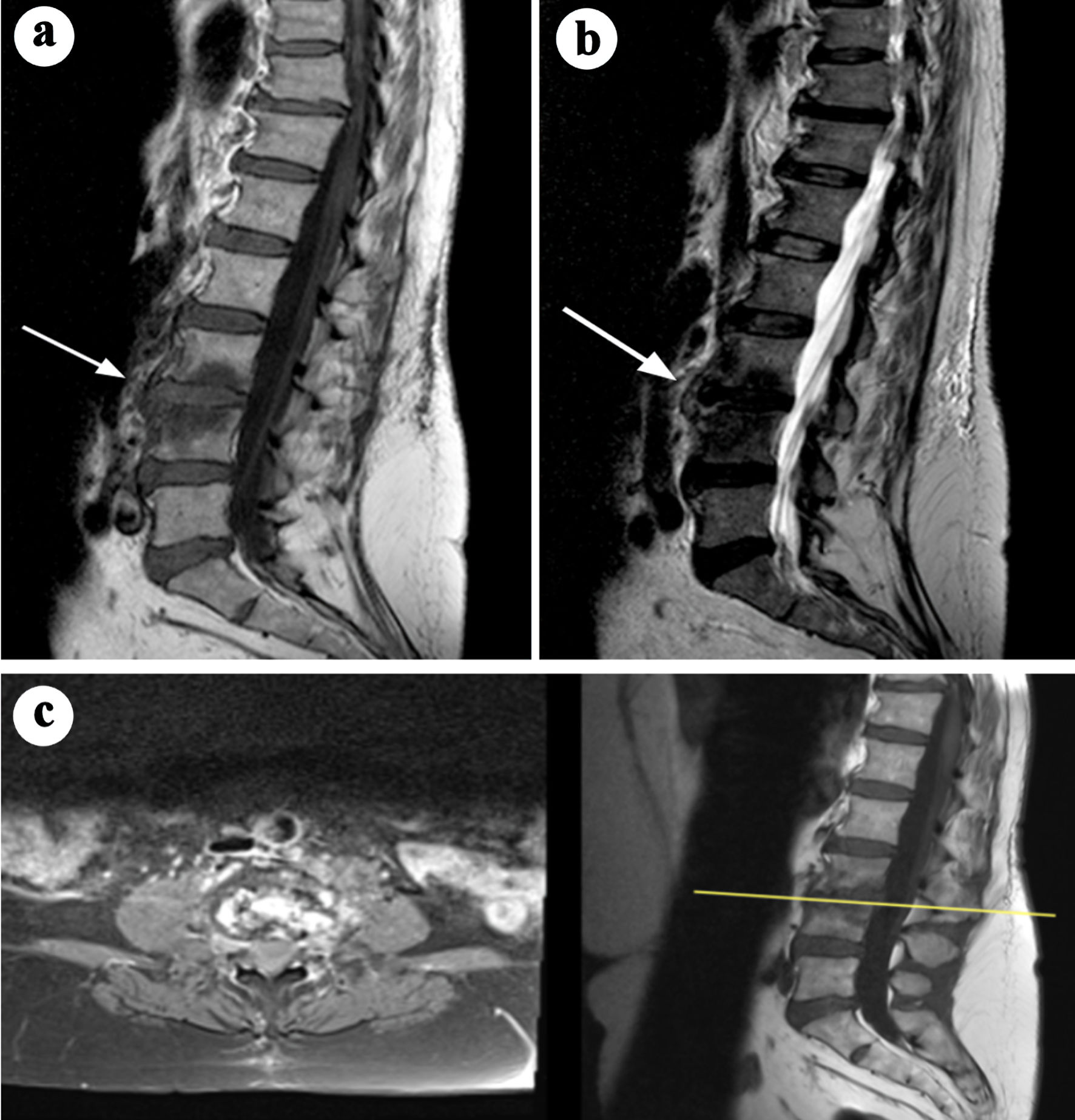
 Click for large image | Figure 4. MRI images demonstrating a spinal epidural abscess with associated prevertebral abscess. (a) Sagittal T2-weighted MRI reveals an anterior epidural abscess (C1-C4) compressing the spinal cord, with a hyperintense T2 signal and evidence of a prevertebral abscess at C3-C4 (arrows). (b) Sagittal T1-weighted MRI shows hypointense signals corresponding to the epidural and prevertebral abscesses, consistent with fluid and inflammatory changes (arrows). Adapted from Egidio de Sousa et al [29] (licensed under CC BY 4.0). MRI: magnetic resonance imaging. |
It is essential to note that while clinical symptoms, positive blood cultures, and imaging findings may be suggestive of SI, they are not definitive. A conclusive diagnosis requires microscopic evaluation of the involved tissue. In cases of negative blood cultures and inconclusive imaging, CT-guided needle biopsy is recommended for confirmation of SI diagnosis and further targeted management [33, 34]. A complete algorithm of diagnostic tests is presented in Figure 5.
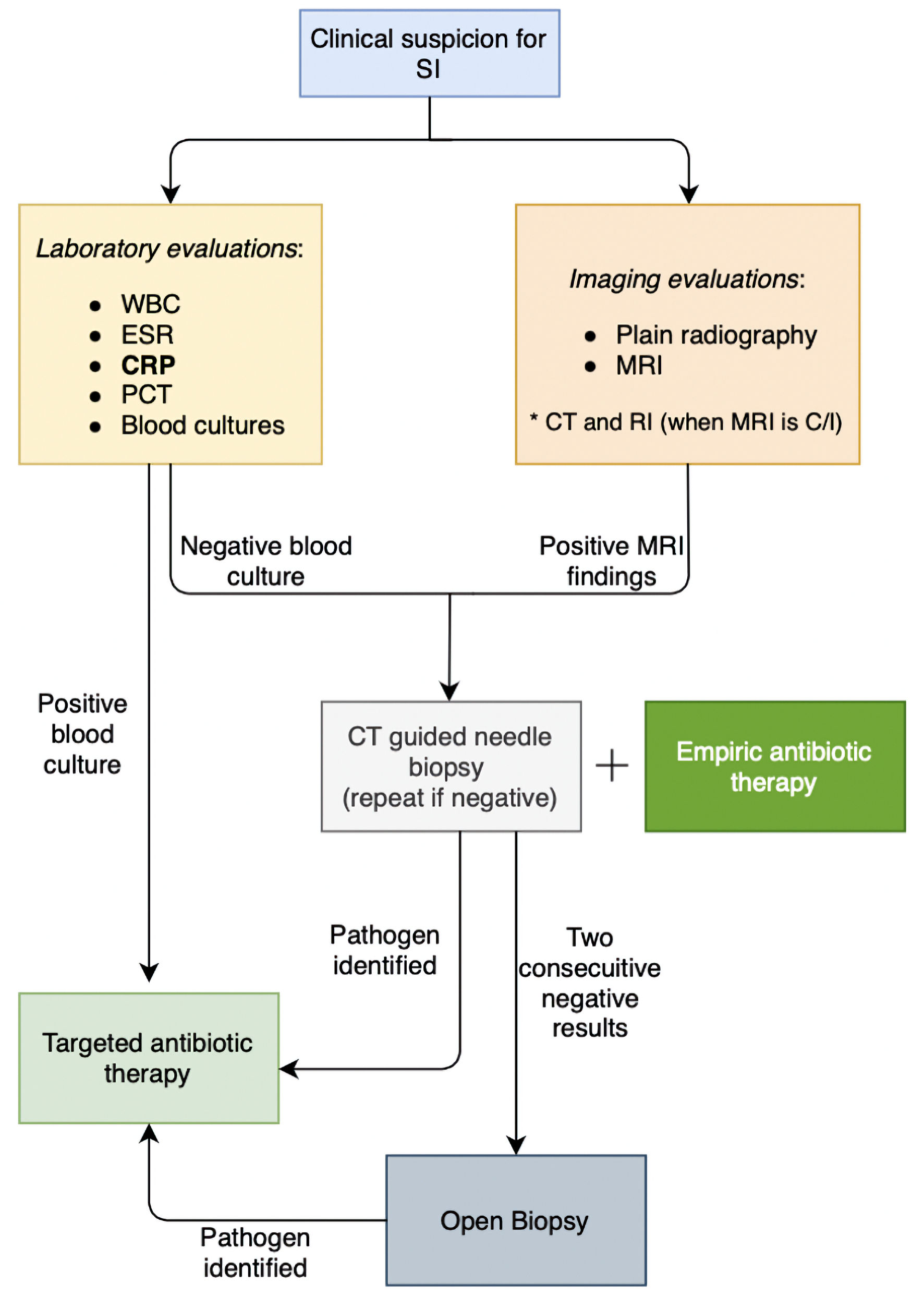 Click for large image | Figure 5. Diagnostic approach to spinal infections. SI: spinal infection; WBC: white blood cells; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; MRI: magnetic resonance imaging; CT: computed tomography; RI: radionuclide imaging; C/I: contraindicated. |
The CT-guided biopsy needed to confirm the diagnosis in certain cases of patients presenting with septicemia should not delay treatment, and prompt empirical antibiotic treatment should be started [19, 29]. Administration of antibiotics before biopsy does not appear to reduce culture sensitivity [35]. When blood cultures are negative and two consecutive needle biopsies are non-diagnostic, an open biopsy is recommended to identify the causative organism [36].
| Management | ▴Top |
The management of SI is guided by several aspects of a patient’s clinical picture. Treatment goals include elimination of the presenting infection, maximal preservation of the affected spinal cord structure, and if needed, the decompression of the spinal canal in the presence of neurological deficits by mass effect. Antibiotic therapy is a mainstay of all treatment approaches but the inclusion of surgical intervention in management is based on the level of osseous involvement, neurological decline, systemic involvement, and response to intravenous antibiotic therapy [9, 37].
Conservative approaches incorporate intravenous antibiotics in combination with some level of immobilization of the spine to aid in symptomatic pain relief and spinal stability [2]. The initial approach should be multidisciplinary with a spinal surgeon and infectious disease physician setting the goals and parameters of management. Key differentiator of treatments is the location of infection and its causative organism. Spondylodiscitis, or infection of the disc space, is most commonly caused by Staphylococcus aureus in Western countries but Mycobacterium tuberculosis is a common pathogen in the developing world [13, 38]. Generally, empiric therapy for spondylodiscitis is only recommended in cases of neurological deterioration with or without sepsis or hemodynamic shock. Once a causative organism has been cultured, treatment should be guided accordingly. Infections caused by oxacillin-susceptible Staphylococcus aureus should be treated with ceftriaxone, cefazolin, or flucloxacillin. In cases of oxacillin resistance, vancomycin remains the mainstay of treatment. Penicillin G and ampicillin are recommended for cases of Enterococcus with vancomycin being indicated in cases of penicillin resistance [19]. The length of antibiotic therapy remains controversial as 6 weeks of therapy was seen to be non-inferior to 12 weeks at 1 year post initiation [39]. The Infectious Disease Society of America recommends 6 weeks of parental or highly bioavailable oral antibiotic therapy but 3 months for patients with Brucella presentations [19].
Surgical indications include neurological deficits, sepsis, presence of intraspinal empyema or abscess greater than 2.5 cm, and failure of conservative therapy. There is also an instability criterion consisting of segmental kyphosis greater than 15°, vertebral body collapse greater than 50%, or translation greater than 5 mm [40]. A common consideration for surgical intervention is advanced age, but older patients have been shown to have similar mortality rates as their younger counterparts albeit with higher complications rates [22]. Spinal intramedullary abscesses are among the rarer infectious presentations of SIs [41]. As illustrated in Figure 6 [41], MRI demonstrates an intramedullary abscess characterized by a hyperintense lesion on T2W imaging with surrounding cord edema and peripheral ring enhancement. Given the lack of standardized treatment guidelines, management remains on a case-by-case basis as far the composition of antibiotic therapy with surgical intervention. A 202-patient case review provided insight into treatment approaches and outcomes. In this analysis, all patients received antibiotic therapy, and 65% underwent surgical drainage. In total, 77% of patients across both treatment groups demonstrated improvement at the 6-month follow-up. In patients who underwent surgical treatment, those operated on within 24 h of diagnosis had a significantly higher chance of being ambulatory at follow-up compared to those who received surgery after 24 h (odds ratio (OR): 4.44; 95% confidence interval (CI): 1.26 - 15.61; P = 0.020) [42].
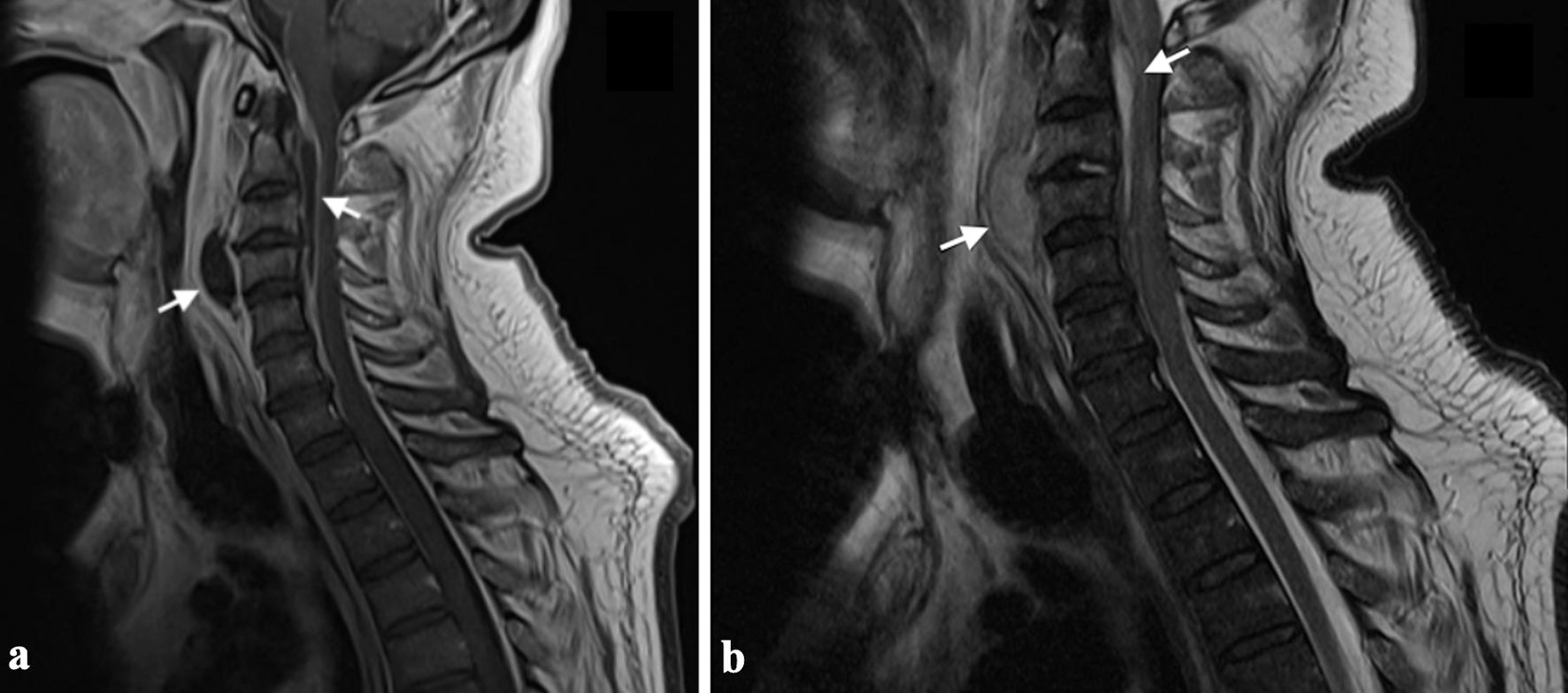
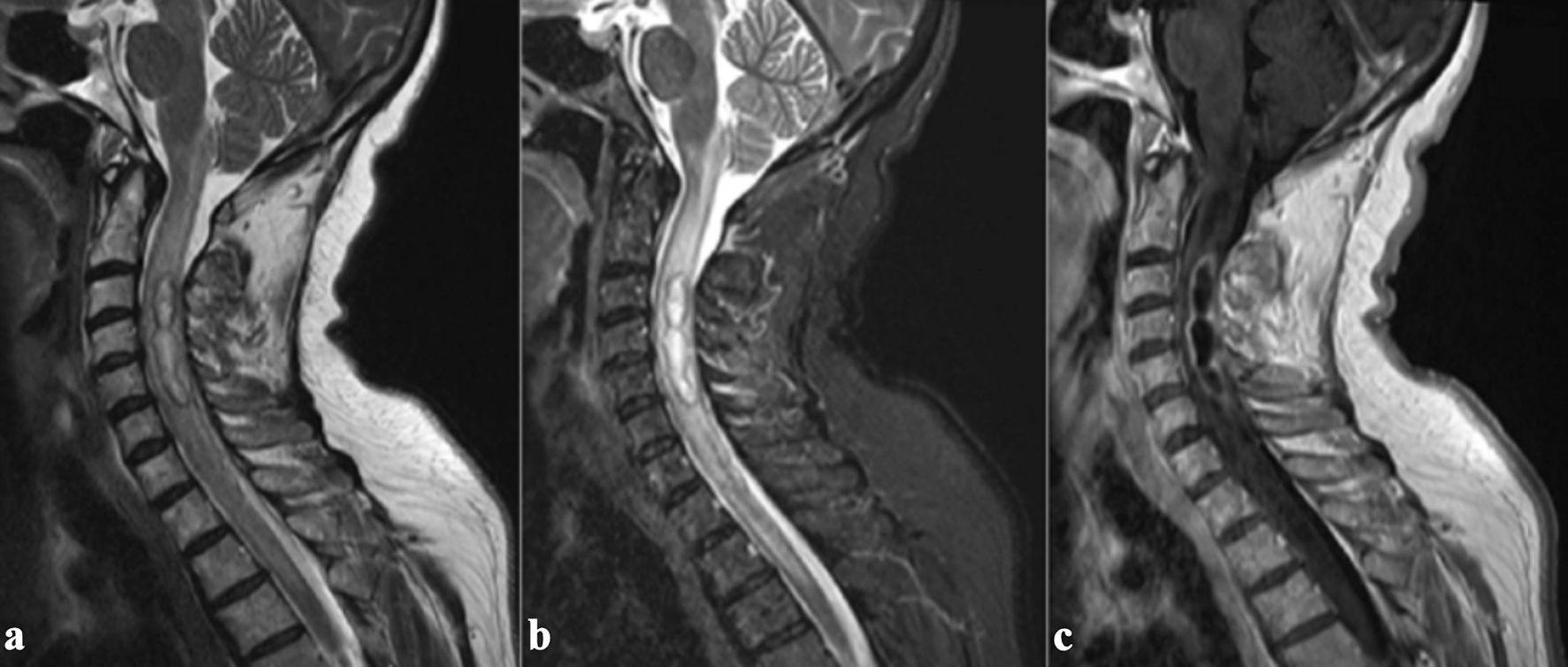 Click for large image | Figure 6. MRI images illustrating the radiological features of an intramedullary abscess in the cervical spinal cord. (a) Sagittal T2-weighted MRI reveals an intramedullary hyperintense lesion within the cervical spinal cord, associated with diffuse core expansion and surrounding edema. (b) Short tau inversion recovery (STIR) sequence further highlights the hyperintense lesion and associated edema. (c) Post-contrast sagittal T1-weighted image demonstrates continuous peripheral ring enhancement. Adapted from Cerecedo-Lopez et al [41] (licensed under CC BY 4.0). MRI: magnetic resonance imaging. |
Septic facet joints are also another rare infection of the spine and often occur as a spread of an already existing infection [29]. MRI findings of septic arthritis in the facet joint are depicted in Figure 7 [28]. One five-patient cases series detailed how four out of five patients went to have complete recovery only with intravenous antibiotics for an average duration of 33.3 days. One patient with recurrent infections did require open facet arthrotomy and paraspinal muscle debridement after intravenous administration of antibiotics, which suggests a possible surgical role for refractory cases [43, 44]. An example of a paraspinal abscess is presented in Figure 8 [45]. A systematic review of septic facet joints in children found that the average antibiotic treatment duration was 5.1 weeks, with compliance to guidelines at 79% for empiric and 62% for targeted antibiotic therapies [29, 46]. Spinal subdural empyema presents a significant risk of neurological compromise necessitating a much lower threshold for surgical intervention [45]. As illustrated in Figure 9 [43], spinal subdural empyema appears as large, loculated fluid collections causing significant spinal cord compression and associated T2 hyperintensity. A narrow indication exists for treatment solely of antibiotic therapy if there is limited neurological deficit, a limited empyema, and the patient responds well early [45]. These patients will require consistent exams and neuroimaging with close supervision [47].
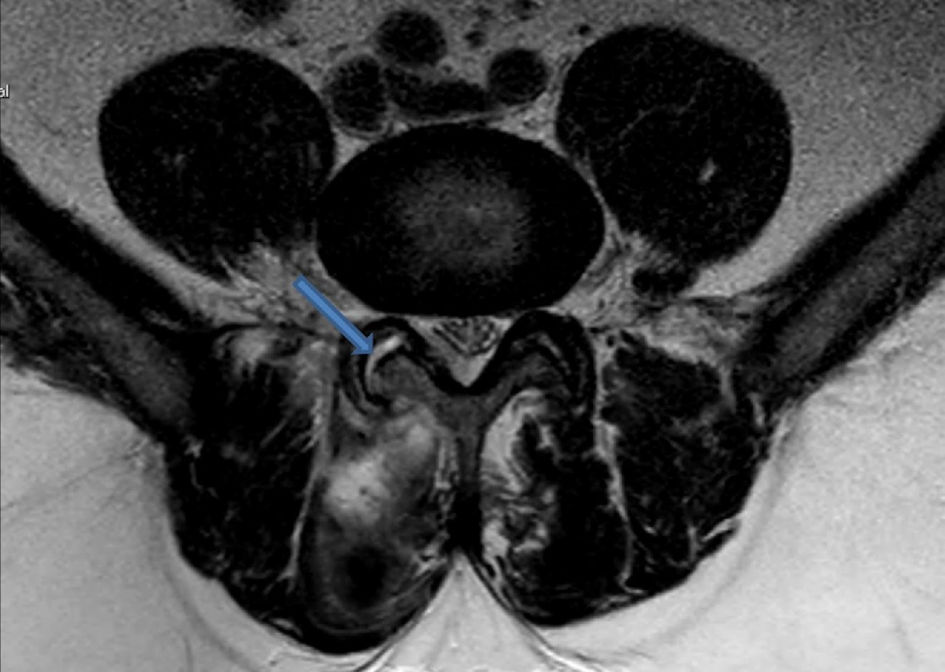 Click for large image | Figure 7. Axial T2-weighted MRI image illustrating fluid collection within the right facet joint, consistent with findings of septic arthritis. The hyperintense signal in the joint space (blue arrow) indicates inflammation. Adapted from Rajeev et al [28] (licensed under CC BY 4.0). MRI: magnetic resonance imaging. |
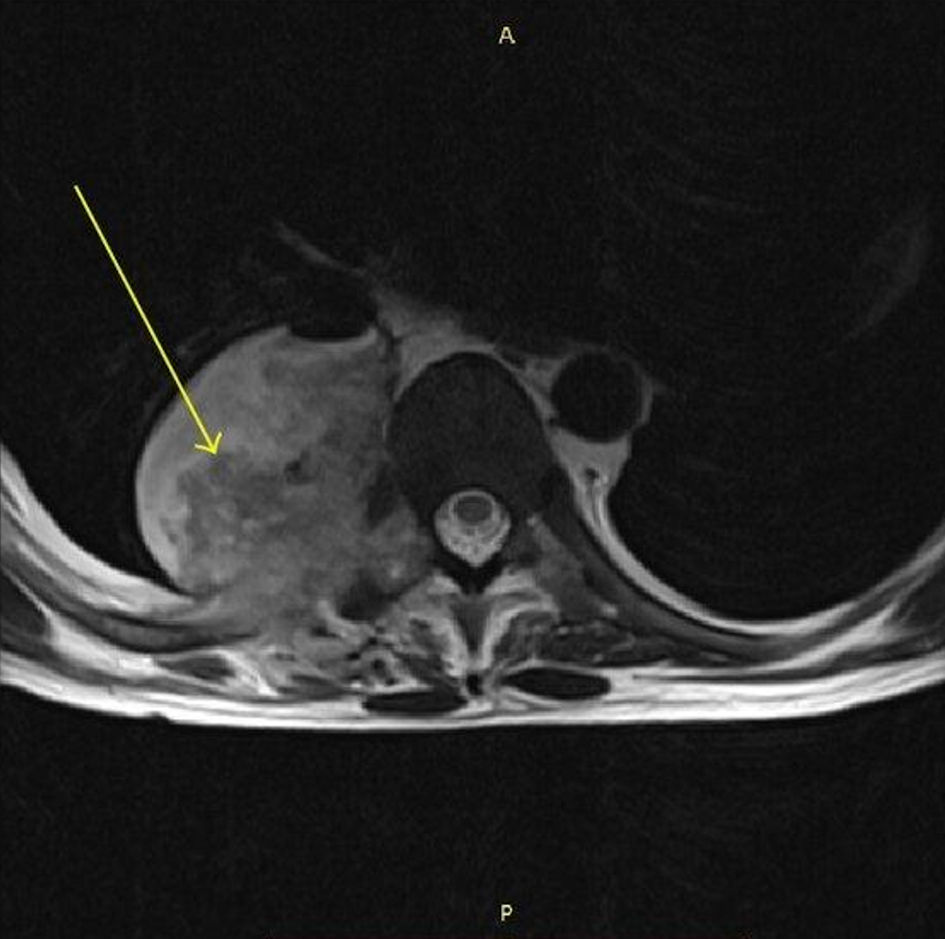 Click for large image | Figure 8. MRI spine (axial T1-weighted) showing a paraspinal abscess on the right side with an air-fluid level (arrow). Adapted from the study of Eswarappa et al [45] (licensed under CC BY 3.0, image modified by the authors to include an annotation (yellow arrow)). MRI: magnetic resonance imaging. |
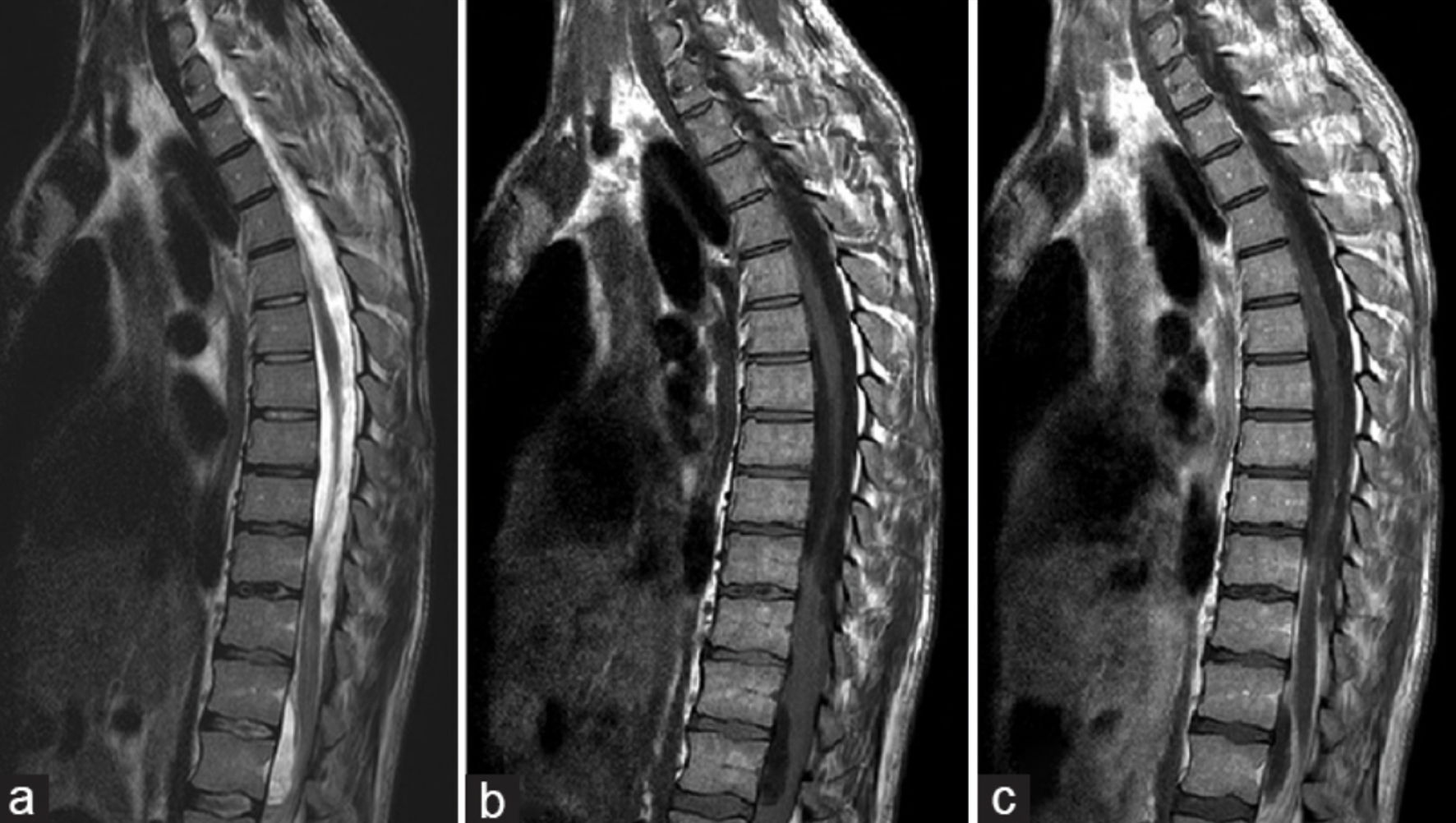 Click for large image | Figure 9. MRI images demonstrating spinal subdural empyema with associated fluid collections. (a) On sagittal T2-weighted imaging, large loculated fluid collections are observed, resulting in ventral compression of the spinal cord between T12 and L2 and dorsal compression extending from T8 to T12, with associated T2 hyperintensity involving the distal thoracic cord and conus at T11-T12. (b) Sagittal T1-weighted MRI without contrast shows anterior displacement of the spinal cord from T9-T12 levels. (c) Sagittal T1-weighted MRI with contrast demonstrates distinct enhancement of fluid collections at T8-T12 and T12-L2, as well as enhancement of the leptomeninges and nerve roots within the cauda equina, findings consistent with inflammation. Adapted from Basheer et al [43] (licensed under CC BY 3.0). MRI: magnetic resonance imaging. |
| Limitations | ▴Top |
While this narrative review provides a comprehensive synthesis of the current literature on acute SIs, certain limitations should be acknowledged. As with all narrative reviews, the lack of formal quality assessment and quantitative data synthesis may introduce some degree of selection bias. However, the inclusion of a wide range of peer-reviewed studies, case reports, and clinical guidelines ensures a robust and diverse perspective. Additionally, variability in diagnostic and treatment approaches across studies reflects real-world practice differences and offers valuable insight into the complexities of managing these conditions. Future research, particularly prospective and systematic investigations, will further strengthen the evidence base and help refine best practices.
| Conclusions | ▴Top |
In conclusion, this narrative review has provided a comprehensive overview of SIs, shedding light on the multifaceted nature of these debilitating conditions. SIs, encompassing various etiologies such as bacterial, fungal, and mycobacterial, present a significant challenge to both patients and healthcare professionals. Through the exploration of epidemiology, clinical manifestations, diagnostic modalities, and treatment options, this review has highlighted the importance of early detection and prompt intervention in managing SIs effectively.
Our analysis underscores the significance of a multidisciplinary approach involving orthopedic surgeons, neurosurgeons, neurologists, infectious disease specialists, radiologists, and microbiologists. It is crucial to tailor treatment strategies to the specific infective agent and consider the individual patient’s clinical status and comorbidities. Advances in imaging techniques, such as MRI and positron emission tomography-computed tomography (PET-CT) scans, have greatly improved our ability to diagnose SIs accurately, allowing for more targeted therapeutic interventions.
Moreover, the emergence of antibiotic-resistant pathogens and the need for vigilant surveillance systems call for continued research efforts in the field of SIs. Future studies should focus on optimizing diagnostic accuracy, refining treatment protocols, and exploring innovative therapeutic modalities, such as minimally invasive surgical techniques and immunomodulatory therapies. Ultimately, this narrative review underscores the significance of a holistic approach to managing SIs, emphasizing the importance of early diagnosis, tailored treatment, and ongoing research to improve patient outcomes and enhance our understanding of these complex conditions.
Acknowledgments
The authors have no acknowledgments to declare.
Financial Disclosure
The authors declare that there was no financial support, sponsorship, or funding received for the completion of this study.
Conflict of Interest
No conflict of interest to report.
Author Contributions
Eti Muharremi, MD: conceptualization, supervision, critical review of the manuscript. Francis Demiraj, DO: literature review, writing - original draft preparation, and editing. Denis Babici, MD: data analysis, interpretation of findings, and manuscript revision. Ayleen Shaban, OMS-III: literature search, data collection, writing - original draft preparation. Tugce Kutuk, MD: critical revision of the manuscript, methodology support. Sujai Nath, MD: final review, approval of the manuscript, and project administration.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Nickerson EK, Sinha R. Vertebral osteomyelitis in adults: an update. Br Med Bull. 2016;117(1):121-138.
doi pubmed - Aljawadi A, Jahangir N, Jeelani A, Ferguson Z, Niazi N, Arnall F, Pillai A. Management of Pyogenic Spinal Infection, review of literature. J Orthop. 2019;16(6):508-512.
doi pubmed - Hadjipavlou AG, Mader JT, Necessary JT, Muffoletto AJ. Hematogenous pyogenic spinal infections and their surgical management. Spine (Phila Pa 1976). 2000;25(13):1668-1679.
doi pubmed - Skaf GS, Domloj NT, Fehlings MG, Bouclaous CH, Sabbagh AS, Kanafani ZA, Kanj SS. Pyogenic spondylodiscitis: an overview. J Infect Public Health. 2010;3(1):5-16.
doi pubmed - Calhoun JH, Manring MM. Adult osteomyelitis. Infect Dis Clin North Am. 2005;19(4):765-786.
doi pubmed - Grammatico L, Baron S, Rusch E, Lepage B, Surer N, Desenclos JC, Besnier JM. Epidemiology of vertebral osteomyelitis (VO) in France: analysis of hospital-discharge data 2002-2003. Epidemiol Infect. 2008;136(5):653-660.
doi pubmed - Mylona E, Samarkos M, Kakalou E, Fanourgiakis P, Skoutelis A. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum. 2009;39(1):10-17.
doi pubmed - Legout L, Assal M, Rohner P, Lew D, Bernard L, Hoffmeyer P. Successful treatment of Candida parapsilosis (fluconazole-resistant) osteomyelitis with caspofungin in a HIV patient. Scand J Infect Dis. 2006;38(8):728-730.
doi pubmed - Lener S, Hartmann S, Barbagallo GMV, Certo F, Thome C, Tschugg A. Management of spinal infection: a review of the literature. Acta Neurochir (Wien). 2018;160(3):487-496.
doi pubmed - Issa K, Diebo BG, Faloon M, Naziri Q, Pourtaheri S, Paulino CB, Emami A. The epidemiology of vertebral osteomyelitis in the United States from 1998 to 2013. Clin Spine Surg. 2018;31(2):E102-E108.
doi pubmed - Gonzalez Herrera G. Spondylodiscitis [Internet]. Radiopaedia.org; 2024.
doi - Di Muzio B. Discitis osteomyelitis [Internet]. Radiopaedia.org; 2018.
doi - Aguiar G. Candida albicans spondylitis [Internet]. Radiopaedia.org; 2019.
doi - Babic M, Simpfendorfer CS. Infections of the spine. Infect Dis Clin North Am. 2017;31(2):279-297.
doi pubmed - Jeong SJ, Choi SW, Youm JY, Kim HW, Ha HG, Yi JS. Microbiology and epidemiology of infectious spinal disease. J Korean Neurosurg Soc. 2014;56(1):21-27.
doi pubmed - Mavrogenis AF, Triantafyllopoulos GK, Kokkinis K, Stefos A, Sipsas NV, Pneumaticos SG. Continuous L3 spondylitis caused by an infected endovascular aortic graft. Surg Infect (Larchmt). 2014;15(6):861-862.
doi pubmed - Megaloikonomos PD, Antoniadou T, Dimopoulos L, Liontos M, Igoumenou V, Panagopoulos GN, Giannitsioti E, et al. Spondylitis transmitted from infected aortic grafts: a review. J Bone Jt Infect. 2017;2(2):96-103.
doi pubmed - Patel AR, Alton TB, Bransford RJ, Lee MJ, Bellabarba CB, Chapman JR. Spinal epidural abscesses: risk factors, medical versus surgical management, a retrospective review of 128 cases. Spine J. 2014;14(2):326-330.
doi pubmed - Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK, Hendershot EF, et al. 2015 Infectious Diseases Society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. 2015;61(6):e26-46.
doi pubmed - Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am. 1997;79(6):874-880.
doi pubmed - Lillie P, Thaker H, Moss P, Baruah J, Cullen L, Taylor D, Barlow G. Healthcare associated discitis in the era of antimicrobial resistance. J Clin Rheumatol. 2008;14(4):234-237.
doi pubmed - Sobottke R, Rollinghoff M, Zarghooni K, Zarghooni K, Schluter-Brust K, Delank KS, Seifert H, et al. Spondylodiscitis in the elderly patient: clinical mid-term results and quality of life. Arch Orthop Trauma Surg. 2010;130(9):1083-1091.
doi pubmed - Gasbarrini A, Boriani L, Salvadori C, Mobarec S, Kreshak J, Nanni C, Zamparini E, et al. Biopsy for suspected spondylodiscitis. Eur Rev Med Pharmacol Sci. 2012;16(Suppl 2):26-34.
pubmed - Lew DP, Waldvogel FA. Osteomyelitis. Lancet. 2004;364(9431):369-379.
doi pubmed - Hovi I, Lamminen A, Salonen O, Raininko R. MR imaging of the lower spine. Differentiation between infectious and malignant disease. Acta Radiol. 1994;35(6):532-540.
pubmed - Gouliouris T, Aliyu SH, Brown NM. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother. 2010;65(Suppl 3):iii11-24.
doi pubmed - Arbelaez A, Restrepo F, Castillo M. Spinal infections: clinical and imaging features. Top Magn Reson Imaging. 2014;23(5):303-314.
doi pubmed - Rajeev A, Choudhry N, Shaikh M, Newby M. Lumbar facet joint septic arthritis presenting atypically as acute abdomen - A case report and review of the literature. Int J Surg Case Rep. 2016;25:43-245.
doi pubmed - Egidio de Sousa I, Brito Monteiro M, Piteira M, Cuco A, Telles Freitas P. Epidural abscess: a cause of back pain that must not be missed. Cureus. 2021;13(4):e14376.
doi pubmed - Love C, Patel M, Lonner BS, Tomas MB, Palestro CJ. Diagnosing spinal osteomyelitis: a comparison of bone and Ga-67 scintigraphy and magnetic resonance imaging. Clin Nucl Med. 2000;25(12):963-977.
doi pubmed - Ohtori S, Koshi T, Yamashita M, Yamauchi K, Inoue G, Suzuki M, Orita S, et al. Surgical versus nonsurgical treatment of selected patients with discogenic low back pain: a small-sized randomized trial. Spine (Phila Pa 1976). 2011;36(5):347-354.
doi pubmed - Hall WA, Graeber A, Cecava ND. Vertebral osteomyelitis. In: StatPearls. Treasure Island (FL) ineligible companies. 2025.
pubmed - Karadimas EJ, Bunger C, Lindblad BE, Hansen ES, Hoy K, Helmig P, Kannerup AS, et al. Spondylodiscitis. A retrospective study of 163 patients. Acta Orthop. 2008;79(5):650-659.
doi pubmed - Turunc T, Demiroglu YZ, Uncu H, Colakoglu S, Arslan H. A comparative analysis of tuberculous, brucellar and pyogenic spontaneous spondylodiscitis patients. J Infect. 2007;55(2):158-163.
doi pubmed - Saravolatz LD, 2nd, Labalo V, Fishbain J, Szpunar S, Johnson LB. Lack of effect of antibiotics on biopsy culture results in vertebral osteomyelitis. Diagn Microbiol Infect Dis. 2018;91(3):273-274.
doi pubmed - Gras G, Buzele R, Parienti JJ, Debiais F, Dinh A, Dupon M, Roblot F, et al. Microbiological diagnosis of vertebral osteomyelitis: relevance of second percutaneous biopsy following initial negative biopsy and limited yield of post-biopsy blood cultures. Eur J Clin Microbiol Infect Dis. 2014;33(3):371-375.
doi pubmed - Duarte RM, Vaccaro AR. Spinal infection: state of the art and management algorithm. Eur Spine J. 2013;22(12):2787-2799.
doi pubmed - Herren C, Jung N, Pishnamaz M, Breuninger M, Siewe J, Sobottke R. Spondylodiscitis: Diagnosis and Treatment Options. Dtsch Arztebl Int. 2017;114(51-52):875-882.
doi pubmed - Bernard L, Dinh A, Ghout I, Simo D, Zeller V, Issartel B, Le Moing V, et al. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet. 2015;385(9971):875-882.
doi pubmed - Dietze DD, Jr., Fessler RG, Jacob RP. Primary reconstruction for spinal infections. J Neurosurg. 1997;86(6):981-989.
doi pubmed - Cerecedo-Lopez CD, Bernstock JD, Dmytriw AA, Chen JA, Chalif JI, Gupta S, Driver J, et al. Spontaneous intramedullary abscesses caused by Streptococcus anginosus: two case reports and review of the literature. BMC Infect Dis. 2022;22(1):141.
doi pubmed - Harrold GK, Ali AS, Berkowitz AL, Bhattacharyya S. Clinical Features and Diagnosis of Intramedullary Spinal Cord Abscess in Adults: A Systematic Review. Neurology. 2023;101(8):e836-e844.
doi pubmed - Basheer A, Macki M, Buraimoh M, Mahmood A. Chronic thoracolumbar subdural empyema: Case report and surgical management. Surg Neurol Int. 2017;8:167.
doi pubmed - Doita M, Nabeshima Y, Nishida K, Fujioka H, Kurosaka M. Septic arthritis of lumbar facet joints without predisposing infection. J Spinal Disord Tech. 2007;20(4):290-295.
doi pubmed - Eswarappa M, Varma PV, Madhyastha R, Reddy S, Gireesh MS, Gurudev KC, Mysorekar VV, et al. Unusual fungal infections in renal transplant recipients. Case Rep Transplant. 2015;2015:292307.
doi pubmed - Cabet S, Perge K, Ouziel A, Lacalm A, Vandergugten S, Guibaud L, Ferry T, et al. Septic Arthritis of Facet Joint in Children: A Systematic Review and a 10-year Consecutive Case Series. Pediatr Infect Dis J. 2021;40(5):411-417.
doi pubmed - Dill SR, Cobbs CG, McDonald CK. Subdural empyema: analysis of 32 cases and review. Clin Infect Dis. 1995;20(2):372-386.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Clinical Infection and Immunity is published by Elmer Press Inc.