| Clinical Infection and Immunity, ISSN 2371-4972 print, 2371-4980 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Clin Infect Immun and Elmer Press Inc |
| Journal website https://cii.elmerpub.com |
Short Communication
Volume 000, Number 000, June 2025, pages 000-000
Interleukin-26 Differentially Modulates Human Macrophage Inflammatory Response to Distinct Mycobacterium tuberculosis Whole Cell Lysates
Jose Barragana, Diana Padillaa, Jorge Cervantesb, c
aDepartment of Translational and Molecular Medicine, Texas Tech University Health Sciences Center, El Paso, TX, USA
bDepartment of Medical Education, Dr. Kiran C. Patel College of Allopathic Medicine, Nova Southeastern University, Fort Lauderdale, FL, USA
cCorresponding Author: Jorge Cervantes, Department of Medical Education, Dr. Kiran C. Patel College of Allopathic Medicine, Nova Southeastern University, Fort Lauderdale, FL 33328, USA
Manuscript submitted April 8, 2025, accepted May 23, 2025, published online June 9, 2025
Short title: IL-26 Modulates Macrophage Response to Mtb
doi: https://doi.org/10.14740/cii507
| Abstract | ▴Top |
Background: Interleukin-26 (IL-26) is an antimicrobial peptide that may contribute to the elimination of intracellular Mycobacterium tuberculosis (Mtb). The aim of the study was to investigate how IL-26 affected the response of human macrophages to Mtb whole cell lysates from different lineages.
Methods: Human macrophages were treated with IL-26 (monomer or dimer) before stimulation with Mtb lysates from Indo-Oceanic, HN878, East-African-Indian, and CDC1551 strains.
Results: Nuclear factor kappa B (NF-κB) activation was similar in untreated cells after stimulation with all lysates, but was diminished in IL-26 monomer-treated macrophages in response to Indo-Oceanic lysates. Macrophage exposure to dimeric IL-26 led to a higher NF-κB activation in response to CDC1551 lysates, but lower to HN878 lysates. No changes were observed in interferon regulatory factor (IRF) activation. Overall, IL-26 modulates NF-κB activation in a strain and confirmation-dependent manner. This modulation influences the macrophage inflammatory response to Mtb. Our results suggest that IL-26 may promote M1 polarization and enhance anti-Mtb immunity through the NF-κB pathway. This is particularly true in response to high-cytokine-inducing strains like CDC1551.
Conclusion: More studies are needed to clarify IL-26’s dual role in inflammation and immune regulation, and to explore its therapeutic potential in tuberculosis (TB) treatment and vaccine strategies.
Keywords: Mycobacterium tuberculosis; Macrophages; IL-26
| Introduction | ▴Top |
The fate of Mycobacterium tuberculosis (Mtb) infection is initially dictated by the macrophage response of the human host [1]. Mtb initially infects and resides intracellularly in macrophages after subverting normal phagolysosome fusion processes [2].
Mtb can be divided into distinct lineages, lineage 1 (Indo-Oceanic lineage), lineage 2 (East Asian), lineage 3 (CAS/Delhi), lineage 4 (Euro-American), lineage 5 (West African 1), and lineage 6 (West African 2) [3]. The modern lineages 2, 3, and 4 consist of Mtb strains responsible for the largest and global epidemics and current pandemics in sub-Saharan Africa, Southeast Asia, and Eastern Europe, while the ancient lineage 1 causes tuberculosis (TB) in the Indo-Oceanic regions and the Philippines [4]. Different lineages are not only associated with distinctive clinical profiles, relapse, and antibiotic resistance, but with the ability to induce a proinflammatory response [5]. Several human and animal studies have shown lineage 2 strains to induce lower levels of proinflammatory T-helper (Th)1 cytokines such as tumor necrosis factor (TNF), interleukin (IL)-12, and interferon (IFN)-γ, which may help, in part, to explain its enhanced virulence [3, 5]. Modern lineages are associated with more severe lung damage, and higher TNF-α levels in patients with TB compared to ancient lineages [6]. Lineage 3 strains induce high levels of TNF and intermediate levels of IL-12, and lineage 4 induces high levels of IL-12 and intermediate levels of TNF [3].
IL-26 is a human antimicrobial peptide, which can activate macrophages and facilitate killing of Mtb [7] and Mycobacterium leprae (M. leprae) [8]. IL-26 is a member of the IL-10 cytokine family [9]. Th1 and Th17 cells might be the major cell sources of IL-26 in the lung [10]. Structurally, IL-26 is a cationic amphipathic protein that exists in both monomeric and dimeric forms. The dimeric conformation is stabilized through non-covalent interactions and has been reported to possess greater potential for forming higher order aggregates. The monomeric form is known to usually elicit a lesser effect on inflammatory response than heterodimer forms. Nevertheless, its priming effect on immune responses cannot be undermined as it enhances the phagocytotic activity of THP-1 derived macrophages against other bacteria in infection cell assays. Further studies are needed to characterize their differences and similarities in their immunomodulatory effects. In vitro studies have shown that IL-26 contributes to the elimination of intracellular Mtb via reactive oxygen species (ROS) production by THP-1 cells [11]. An enhanced IL-26 secretion mediated by cytokines like IL-1b has been shown in Th17 cells upon M. leprae stimulation. Such induction is more rapid than IFN-γ and IL-17A-mediated activation [12]. Such mediation adds to the evidence of how cytokines like IL-1b could influence Th17 response and function [13].
Human reports on the role of IL-26 in active TB have been somewhat contradictory. IL-26 transcription has been shown to be upregulated in peripheral blood mononuclear cells of active TB patients compared to healthy controls [11]. At the same time, IL-26 transcription is down-regulated in infected monocytes from TB patients [14]. Furthermore, plasma [11] and serum [14] levels of IL-26 levels of TB patients were markedly lower than those of healthy controls. This reduction is mediated by Mtb, as IL-26 levels of non-infected human monocytes are greatly diminished after Mtb infection [14].
We here aimed to investigate how monomeric and dimeric forms of IL-26 affect the response of human macrophages to Mtb whole cell lysates from different lineages, focusing on NF-κB activation and macrophage polarization.
| Materials and Methods | ▴Top |
Cell assays
THP-1-Dual cells (InvivoGen), which is a human monocytic cell line with NF-κB-SEAP and IRF-Lucia luciferase reporters, were utilized in these studies. Cells were cultured in Roswell Park Memorial Institute (RPMI) 1640, 2 mM L-glutamine, 25 mM N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid (HEPES), 10% heat-inactivated fetal bovine serum (FBS), and penicillin-streptomycin (100 U/mL-100 µg/mL) media. Cells were transformed into macrophages by the addition of 5 ng/mL of phorbol 12-myristate 13-acetate (PMA) for 72 h, and then treated with 1 µM of IL-26 monomer or IL-26 dimer (R&D Systems) for 24 h. Following treatment, cells were stimulated with Mycobacterium lysates from the following Mtb strains: HN878, Indo-Oceanic, East African, CDC1551, or M. bovis (AF2122/97, BEI Resources) for 24 h. Lysates were prepared by BEI Resources through the following procedures: a culture was grown to late log phase in glycerol-alanine-salts medium and inactivated by gamma irradiation. Cells were suspended in PBS buffer containing 8 mM ethylenediaminetetraacetic acid (EDTA), proteinase inhibitors, DNase, and RNase and disrupted by French Press until approximately 90% breakage was obtained. The lysate was centrifuged to pellet the unbroken cells and the cleared supernatant was removed. Lipopolysaccharide (LPS) and lipoarabinomannan (LAM) were used as controls as they are known to have overlapping effects in macrophage activation. Supernatants from condition were collected and reporter activation was evaluated in accordance with the manufacturer’s instructions (InvivoGen). Absorbance at an optical density (OD) of 620 nm was read to measure NF-κB activation after addition of the QUANTI-Blue reagent (InvivoGen), as per manufacturer’s instructions. Luciferase activity was read through the luminometer function and expressed as relative light units (RLUs). A response ratio, compared to untreated and unstimulated cells, was calculated for both reporter systems. All measurements were read using a Synergy-HTX plate reader (Agilent) after addition of the QUANTI-Luc reagent.
STRING protein-protein interaction network
A protein-protein interaction network analysis was conducted through the STRING (Search Tool for the Retrieval of Interacting Genes/Proteins) database (version 11.5). IL-26 was matched to known and predicted interactions derived from various sources. These included experimental data, computational predictions, co-occurrence, co-expression, gene fusion, neighborhood, and text mining. Only known interactions to Homo sapiens were considered at a confidence of 0.400 and cut-off at no more than 10 interactions.
| Results | ▴Top |
NF-κB activation was similar in untreated cells stimulated with Mtb lysates from all strains (Fig. 1a, light bars). This response was diminished in IL-26 monomer-treated macrophages upon stimulation with lysate from Mtb Indo-Oceanic (lineage 1). In macrophages exposed to dimeric IL-26, the NF-κB was statistically significantly higher in response to stimulation with Mtb lysates from CDC1551 strain (lineage 4), but lower in response to lysates from HN878 strain (lineage 2) (Fig. 1a, darker bars). There was no change in the NF-κB response of IL-26-treated cells to lysates from Mtb East-African-Indian strain (lineage 3), M. bovis lysate, purified LAM, or LPS.
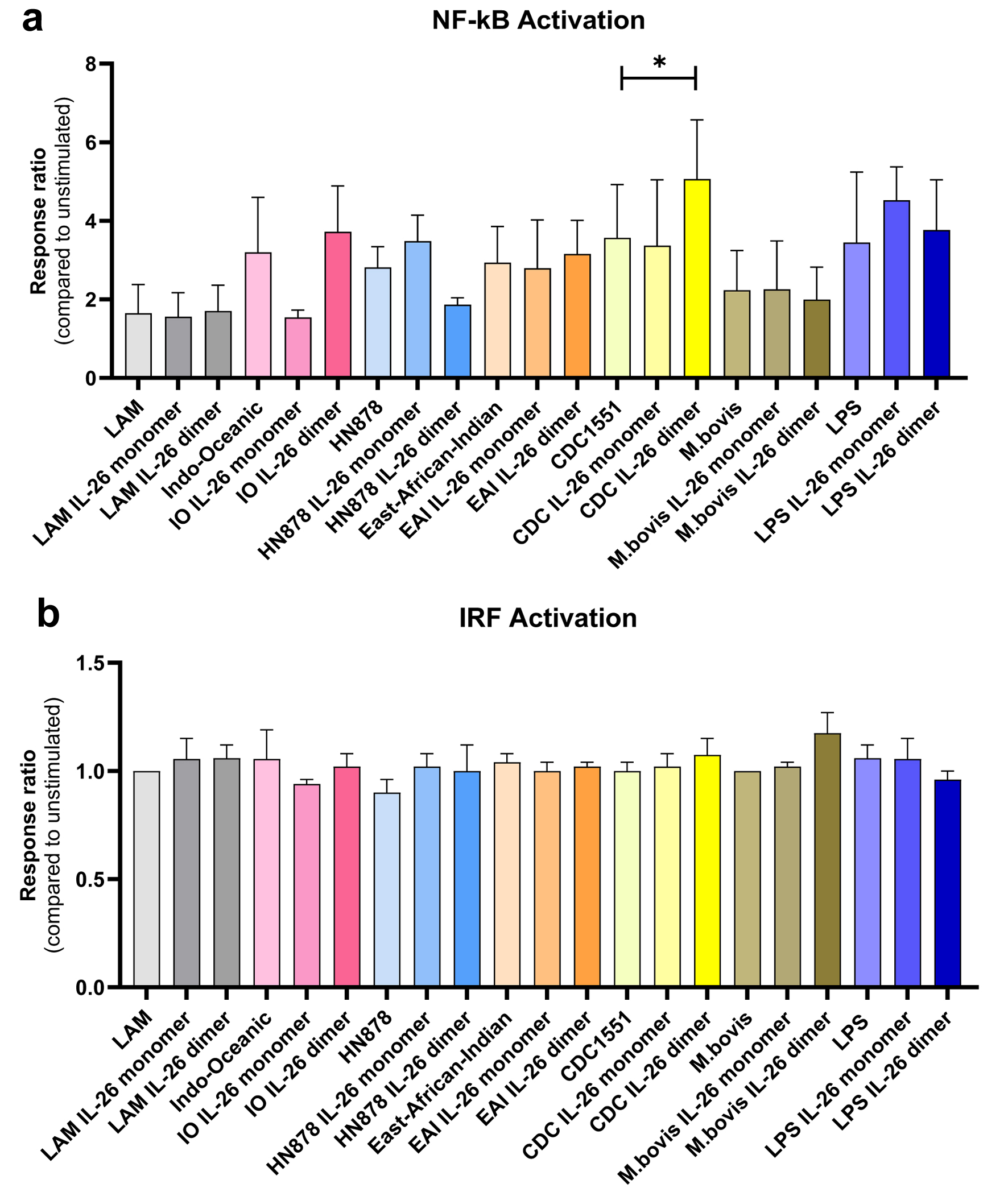 Click for large image | Figure 1. (a) NF-κB and (b) IRF activity in human macrophages in response to Mtb lysate stimulation from different lineages: Indo-Oceanic (lineage 1), HN878 (lineage 2, also known as East Asian), East-African-Indian (lineage 3), CDC1551 (lineage 4), and M. bovis. Lipoarabinomannan (LAM) and lipopolysaccharide (LPS) were used as controls. Untreated: lighter color. IL-26 monomer-treated: medium color. IL-26 dimer-treated: darker color. *P < 0.05 (Wilcoxon matched-pairs signed rank test). Data of three independent experiments. IRF: interferon regulatory factor; Mtb: Mycobacterium tuberculosis; NF-κB: nuclear factor kappa B. |
All Mtb lysates exerted a minimal activation of interferon regulatory factor (IRF) in macrophages, irrespective if they were treated or not with IL-26 (Fig. 1b).
As there is very little literature on the effect of IL-26 in macrophages [11, 15], we performed a predictive protein-protein interaction network analysis. IL-26 proinflammatory network places it within other IL-10 family functional partners. These included IL-19, IL-22, and IL-24. In addition, the STRING database network pointed out interactions between IL-26 and IL-10RB, IL-20RA, IL-20RB, IL22RA1, IL22RA1, STAT3, and STAT1 (Fig. 2a). Along with these predictions, Gene Ontology enrichment analysis associates IL-26 with cytokine-mediated signaling pathway, regulation of receptor signaling pathway via JAK-STAT and inflammatory responses (Fig. 2b).
 Click for large image | Figure 2. (a) IL-26 proinflammatory network and (b) predicted function. IL-26: interleukin-26. |
| Discussion | ▴Top |
Modern Mtb lineages are associated with high TNF-α levels and increase the risk of having severe lung damage three times among TB patients [6]. Nevertheless, the NF-κB activation was similar in macrophages stimulated with Mtb lysates from all the different strains.
Recent knowledge has demonstrated a key role of IL-26 expression in TB patients and its regulatory effect in macrophage polarization and intracellular elimination of Mtb [11]. Therefore, we aimed to explore how human macrophage immune responses to ligands present in lysates from different Mtb lineages were affected by IL-26. We observed an increased proinflammatory response, measured as NF-κB activation, only to Mtb lysates from CDC1551 strain in IL-26 dimer-treated human macrophages. This is in line with reported heightened expressions of CD80, TNF-α, and iNOS in THP1 cells treated with IL-26 [11]. NF-κB crucially assists in keeping a granuloma [16], and Mtb load contained [17], as granuloma formation is dependent on proinflammatory mediators such as TNF-α [16]. Nevertheless, IL-26 dimer-treated cells showed a decreased NF-κB activation upon stimulation with HN878 lysates. CDC1551 is a high cytokine inductor, while HN878 has a low cytokine induction but high mortality [18]. NF-κB subunit p65 transcription and translocation is higher in cells infected with Mtb CDC1551 compared to HN878 strain [19]. Even DNA from HN878 appears to be weak as a ligand, compared to other strains [20]. Treatment with IL-26 monomer also lowered NF-κB activation in response to lysates from the Indo-Oceanic strain (lineage 1).
The differential response to monomeric and dimeric IL-26 treatment suggests possible distinct modes of action of IL-26 on macrophages [15]. IL-26 is a cationic amphoteric protein that can not only dimerize but also form higher degree dimers. Both the monomer and dimer conformations can bind to the dimeric IL-26 receptor [21]. Therefore, both possess the potential to be biologically active. It is unclear if one form is more active than the other and more studies are needed to confirm the activity.
IL-26 may induce M1 macrophage polarization [11] by activating NF-κB pathways. Despite IL-26 being a member of the IL-10 and IL-20 cytokine family [9], it does not induce M2 macrophage polarization [11]. Since it is mainly secreted by immune cells like Th17, monocytes, and macrophages [14], it is considered a proinflammatory cytokine [9, 22]. Curiously, treatment of peripheral blood mononuclear cells (PBMCs) with IL-26 has shown to have no effect on the amount of produced TNF-α, nor other NF-κB-dependent cytokines like IL-12. Nevertheless, it can reduce the amounts of IL-2 receptors (IL-2R) [16] and IL-2 [14], which is a major key player in T-cell activation and is critical for Mtb elimination. All this suggests an inhibitory effect on the immune response of IL-26, similar to that described for IL-10, and may constitute another Mtb immune evasion mechanism that mediates long-term infections in the lung.
The lack of IRF activation, associated with production of type I IFNs observed in active TB, may be due to an absence of endosomal Toll-like receptor (TLR) pathogen-associated molecular patterns (PAMPs) present in live mycobacteria but not in our lysates. DNA from these strains is able to induce IRF activation when delivered to the endosomal compartment [20]. Type I IFN plays a role in diminishing the host defense against Mtb by attenuating T-cell activation [23]. Type I IFN from macrophages also influences neutrophils migrating into the granuloma, enabling the release of neutrophil extracellular traps (NETs) associated with necrosis and caseation [24]. IL-26 plays a key role in attracting neutrophils to the lung [25]. Neutrophils infiltration is associated with active TB [26] and disruption of the granuloma architecture [27].
It is unknown to what extent the differences in NF-κB activation observed upon IL-26 pretreatment would impact on cytokine production in human macrophages. Further investigation is needed to assess these effects. Nevertheless, the predictive protein network analysis suggests that IL-26 elicits a proinflammatory response to Mtb in human macrophages through a JAK/STAT/NF-κB axis. IL-26 levels correlate with active TB disease, as well as other granulomatous forms of mycobacterial diseases like leprosy. In leprosy, IL-26 is more strongly expressed in lesions from the tuberculoid form of the disease, associated with M1 macrophage polarization [28], compared to lepromatous leprosy, which is associated with M2 polarizations [8]. The modulating effects of IL-26 on macrophage polarization towards M1 phenotype and now its activation of NF-κB response could enhance Th1 and cytotoxic T-cell responses, which are essential components for effective TB immunity.
Putting altogether, IL-26 proinflammatory responses could boost M1 macrophage polarization and therefore enhance Th1 and cytotoxic T-cell responses. At the same time, it may exert a modulatory/immunosuppressive effect on type I IFN, which can diminish host defense against Mtb and is associated with active disease. This dual effect can offer therapeutic advantages during active infection treatment or vaccination. Therefore, selectively targeting IL-26 signaling through its monomeric or dimeric forms can be an orchestrated strategy to either amplify host defenses mechanisms or suppress damaging inflammation. In this context, further studies could help streamline the potential role of IL-26 as an adjuvant to TB therapy or vaccines.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
JC conceptualized the project. JB and DP performed the experiments. JB, DP, and JC analyzed the data. JB and JC wrote the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
IRF: interferon regulatory factor; Mtb: Mycobacterium tuberculosis; NF-κB: nuclear factor kappa B; TNF-α: tumor necrosis factor-alpha
| References | ▴Top |
- Cooper AM, Mayer-Barber KD, Sher A. Role of innate cytokines in mycobacterial infection. Mucosal Immunol. 2011;4(3):252-260.
doi pubmed - Verrall AJ, Netea MG, Alisjahbana B, Hill PC, van Crevel R. Early clearance of Mycobacterium tuberculosis: a new frontier in prevention. Immunology. 2014;141(4):506-513.
doi pubmed - Sarkar R, Lenders L, Wilkinson KA, Wilkinson RJ, Nicol MP. Modern lineages of Mycobacterium tuberculosis exhibit lineage-specific patterns of growth and cytokine induction in human monocyte-derived macrophages. PLoS One. 2012;7(8):e43170.
doi pubmed - Coscolla M, Gagneux S. Consequences of genomic diversity in Mycobacterium tuberculosis. Semin Immunol. 2014;26(6):431-444.
doi pubmed - He C, Cheng X, Kaisaier A, Wan J, Luo S, Ren J, Sha Y, et al. Effects of Mycobacterium tuberculosis lineages and regions of difference (RD) virulence gene variation on tuberculosis recurrence. Ann Transl Med. 2022;10(2):49.
doi pubmed - Amin M, Yanti B, Harapan H, Mertaniasih NM. The role of Mycobacterium tuberculosis lineages on lung tissue damage and TNF-α level among tuberculosis patients, Indonesia. Clinical Epidemiology and Global Health. 2019;7(3):263-267.
doi - Hawerkamp HC, van Geelen L, Korte J, Di Domizio J, Swidergall M, Momin AA, Guzman-Vega FJ, et al. Interleukin-26 activates macrophages and facilitates killing of Mycobacterium tuberculosis. Sci Rep. 2020;10(1):17178.
doi pubmed - Dang AT, Teles RM, Weiss DI, Parvatiyar K, Sarno EN, Ochoa MT, Cheng G, et al. IL-26 contributes to host defense against intracellular bacteria. J Clin Invest. 2019;129(5):1926-1939.
doi pubmed - Gowhari Shabgah A, Abdelbasset WK, Sulaiman Rahman H, Bokov DO, Suksatan W, Thangavelu L, Ahmadi M, et al. A comprehensive review of IL-26 to pave a new way for a profound understanding of the pathobiology of cancer, inflammatory diseases and infections. Immunology. 2022;165(1):44-60.
doi pubmed - Zhang M, Niu YR, Liu JY, Wei XS, Wang XR, Ye LL, Peng WB, et al. Interleukin-26 upregulates interleukin-22 production by human CD4(+) T cells in tuberculous pleurisy. J Mol Med (Berl). 2019;97(5):619-631.
doi pubmed - Huang K, Zhou H, Chen M, Chen R, Wang X, Chen Q, Shi Z, et al. Interleukin-26 expression in tuberculosis disease and its regulatory effect in macrophage polarization and intracellular elimination of Mycobacterium tuberculosis. Front Cell Infect Microbiol. 2024;14:1455819.
doi pubmed - Weiss DI, Ma F, Merleev AA, Maverakis E, Gilliet M, Balin SJ, Bryson BD, et al. IL-1beta induces the rapid secretion of the antimicrobial protein IL-26 from Th17 cells. J Immunol. 2019;203(4):911-921.
doi pubmed - Lasiglie D, Traggiai E, Federici S, Alessio M, Buoncompagni A, Accogli A, Chiesa S, et al. Role of IL-1 beta in the development of human T(H)17 cells: lesson from NLPR3 mutated patients. PLoS One. 2011;6(5):e20014.
doi pubmed - Guerra-Laso JM, Raposo-Garcia S, Garcia-Garcia S, Diez-Tascon C, Rivero-Lezcano OM. Microarray analysis of Mycobacterium tuberculosis-infected monocytes reveals IL26 as a new candidate gene for tuberculosis susceptibility. Immunology. 2015;144(2):291-301.
doi pubmed - Hirsh J, Kositangool P, Shah A, Radwan Y, Padilla D, Barragan J, Cervantes J. IL-26 mediated human cell activation and antimicrobial activity against Borrelia burgdorferi. Curr Res Microb Sci. 2020;1:30-36.
doi pubmed - Poladian N, Orujyan D, Narinyan W, Oganyan AK, Navasardyan I, Velpuri P, Chorbajian A, et al. Role of NF-kappaB during Mycobacterium tuberculosis Infection. Int J Mol Sci. 2023;24(2):1772.
doi pubmed - Bai X, Feldman NE, Chmura K, Ovrutsky AR, Su WL, Griffin L, Pyeon D, et al. Inhibition of nuclear factor-kappa B activation decreases survival of Mycobacterium tuberculosis in human macrophages. PLoS One. 2013;8(4):e61925.
doi pubmed - Manca C, Tsenova L, Bergtold A, Freeman S, Tovey M, Musser JM, Barry CE, 3rd, et al. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha /beta. Proc Natl Acad Sci U S A. 2001;98(10):5752-5757.
doi pubmed - Ranjbar S, Boshoff HI, Mulder A, Siddiqi N, Rubin EJ, Goldfeld AE. HIV-1 replication is differentially regulated by distinct clinical strains of Mycobacterium tuberculosis. PLoS One. 2009;4(7):e6116.
doi pubmed - Cervantes JL, Oak E, Garcia J, Liu H, Lorenzini PA, Batra D, Chhabra A, et al. Vitamin D modulates human macrophage response to Mycobacterium tuberculosis DNA. Tuberculosis (Edinb). 2019;116S:S131-S137.
doi pubmed - Meller S, Di Domizio J, Voo KS, Friedrich HC, Chamilos G, Ganguly D, Conrad C, et al. T(H)17 cells promote microbial killing and innate immune sensing of DNA via interleukin 26. Nat Immunol. 2015;16(9):970-979.
doi pubmed - Larochette V, Miot C, Poli C, Beaumont E, Roingeard P, Fickenscher H, Jeannin P, et al. IL-26, a cytokine with roles in extracellular DNA-induced inflammation and microbial defense. Front Immunol. 2019;10:204.
doi pubmed - Shanmuganathan G, Orujyan D, Narinyan W, Poladian N, Dhama S, Parthasarathy A, Ha A, et al. Role of interferons in mycobacterium tuberculosis infection. Clin Pract. 2022;12(5):788-796.
doi pubmed - Chowdhury CS, Kinsella RL, McNehlan ME, Naik SK, Lane DS, Talukdar P, Smirnov A, et al. Type I IFN-mediated NET release promotes Mycobacterium tuberculosis replication and is associated with granuloma caseation. Cell Host Microbe. 2024;32(12):2092-2111.e2097.
doi pubmed - Griffiths KL, Khader SA. Bringing in the cavalry: IL-26 mediates neutrophil recruitment to the lungs. Am J Respir Crit Care Med. 2014;190(10):1079-1080.
doi pubmed - Muefong CN, Sutherland JS. Neutrophils in tuberculosis-associated inflammation and lung pathology. Front Immunol. 2020;11:962.
doi pubmed - Hult C, Mattila JT, Gideon HP, Linderman JJ, Kirschner DE. Neutrophil dynamics affect mycobacterium tuberculosis granuloma outcomes and dissemination. Front Immunol. 2021;12:712457.
doi pubmed - Marin A, Van Huss K, Corbett J, Kim S, Mohl J, Hong BY, Cervantes J. Human macrophage polarization in the response to Mycobacterium leprae genomic DNA. Curr Res Microb Sci. 2021;2:100015.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Clinical Infection and Immunity is published by Elmer Press Inc.