| Clinical Infection and Immunity, ISSN 2371-4972 print, 2371-4980 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Clin Infect Immun and Elmer Press Inc |
| Journal website https://cii.elmerpub.com |
Editorial
Volume 000, Number 000, April 2025, pages 000-000
Collinsella and Prevotella: Mixed Systemic Actions of a Dynamic Duo
Victoria K. Nguyena, Jorge Cervantesb, c
aMedical City Fort Worth, Fort Worth, TX, USA
bDr. Kiran C. Patel College of Allopathic Medicine, Nova Southeastern University, Fort Lauderdale, FL, USA
cCorresponding Author: Jorge Cervantes, Dr. Kiran C. Patel College of Allopathic Medicine, Nova Southeastern University, Fort Lauderdale, FL, USA
Manuscript submitted April 1, 2025, accepted April 9, 2025, published online April 16, 2025
Short title: Collinsella and Prevotella
doi: https://doi.org/10.14740/cii506
The human microbiome is a complex and dynamic ecosystem with trillions of microorganisms that play a critical role in shaping our health. Among these, Collinsella and Prevotella are two significant genera of commensal bacteria with multifaceted roles that range from beneficial to harmful, depending on strain-specific traits, host physiology, and environmental influences [1]. Given the crucial roles of commensal bacteria in host metabolism, gut dysbiosis can lead to serious health implications in the host, including metabolic disorders and inflammatory diseases [2]. Understanding the duality of Collinsella and Prevotella is essential for advancing microbiome research and developing targeted therapies.
Members of the Clostridia class, organisms from the genus Collinsella are bacteria that play a role in the development of the intestinal microbiota. The genus Prevotella belongs to the order Bacteroidales, and is also considered beneficial due to its abundance in healthy intestinal microbiota and association with plant-rich diets [3]. Collinsella is abundant in the intestinal microbiota of Japanese [4], and along with Prevotella in Indian population [5]. In contrast, populations with westernized diets, characterized by low fiber and high fat, harbor lower levels of Prevotella [6, 7]. Fiber consumption increases the abundance of these bacteria in the gastrointestinal (GI) tract [8]. Intake of whole-grain and fiber-rich foods increases the relative abundance of Collinsella certain species of Prevotella, such as P. tannerae [9]. Additionally, specific dietary components can selectively influence microbial populations. For example, Collinsella has been found to be more abundant in groups consuming milk compared to yogurt [10].
In the GI tract, both Collinsella and Prevotella produce short-chain fatty acids, which play a critical role in gut health [11] (Table 1). Collinsella is a known degrader of common dietary fibers and has the ability to produce butyric acid [11, 12]. Butyrate promotes GI barrier integrity, reduces inflammation, and enhances resistance to pathogens [11]. The difference in metabolism is at the species levels for both of these genera, leading to divergent outcomes in different contexts [12, 13]. Certain strains, such as Collinsella aerofaciens TF06-26, produce butyrate and degrade dietary fibers, supporting gut health and reducing inflammation [14]. Other strains are associated with insulin resistance and cardiovascular complications (CVCs). For example, Collinsella abundance has been found to correlate positively with insulin levels, while showing a negative correlation with dietary fiber intake among pregnant women [15]. Additionally, Collinsella was found to be associated with CVC risk in type 2 diabetes patients [14]. In patients with CVCs, the abundance of butyrate-producing strains like C. aerofaciens TF06-26 is reduced [14].
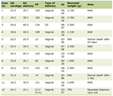 Click to view | Table 1. Microbiological Characteristics of Collinsella and Prevotella |
Alterations in commensal bacteria can contribute to the development of various diseases. Atherosclerosis is associated with the increased abundance of Collinsella, Peptococcaceae, and Prevotella [16, 17]. Collinsella is a TMA-producing bacterium that can contribute to atherogenic effects after being converted to TMA-oxide (TMAO) in the liver [14]. Similarly, atherosclerosis is associated with the increased abundance of Prevotella due to formation of TMAO [16, 17]. Prevotella is significantly decreased in diabetic patients [18], along with an increase of other genera like Clostridium, Bacteroides, and Veillonella. A recent study evaluating changes in gut microbiota during a weight loss program for obese individuals with type 2 diabetes observed a decrease in the levels of Collinsella [19]. An increase in Collinsella abundance could be used as a predictive marker of response to probiotic treatment efficacy in non-constipated irritable bowel syndrome [20].
The microbiota inhabiting the oral cavity is relatively identical to the microbiota that inhabits the lungs, with bacteria traveling from the oral cavity to the lungs through the respiratory tract via migration or microaspiration [21]. Alterations in the oral microbiota could affect the severity of coronavirus disease 2019 (COVID-19) [22]. They exert this action through immunomodulatory role of the oral microbiota in the response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by alveolar macrophages [23]. Intestinal Collinsella was shown to mitigate SARS-CoV-2 infection and exacerbation of COVID-19 [24], as it was negatively correlated with COVID-19 mortality in 10 countries. Prevotella differentially regulates the inflammatory response of human monocytes to SARS-CoV-2 spike glycoproteins [25]. This exacerbated innate immune response translates clinically into severe COVID-19 [26].
One important aspect to consider is that the risks and benefits from these two genera come from differences at the species level [27, 28]. Prevotella species are found at multiple mucosal sites, including the respiratory system, oral cavity, and GI tract. Evidence revealed beneficial effects of some Prevotella strains in the gut such as not only improving cardiovascular disease (CVD) risk factor profile and glucose metabolism, but also pathobiontic properties of some strains which promoted diseases like metabolic syndrome, obesity, inflammatory bowel disease or other inflammatory diseases such as rheumatoid arthritis [29]. Oral administration of Prevotella histicola to arthritis-susceptible mice reduced the incidence and severity of the disease [30]. This same species suppresses multiple sclerosis in animal models through modulation of systemic immune responses [31, 32]. P. histicola decreases the inflammatory activity of Th17 cells and increases the activity of regulatory T cells. On the other hand, patients with rheumatoid arthritis (RA) show increased level of enteric Prevotella copri [33], and autoantigens in these patients exhibit high homology to Prevotella-associated peptides [34]. This phenomenon of molecular mimicry of RA-associated antigens by the gut microbiota has also been reported in a metagenomic study of RA patients [35].
Three mechanisms by which microbiota might contribute to RA pathogenesis are proposed: inflammatory responses (P. copri and Lactobacillus), molecular mimicry (P. copri), and loss of intestinal barrier integrity (Collinsella) [36]. The abundance of Collinsella and Bifidobacterium is increased in RA patients compared with controls [37]. Another study showed that Collinsella, along with Eggerthella, and Faecalibacterium, segregated with RA. The abundance of Collinsella correlated strongly with high levels of alpha-aminoadipic acid and asparagine as well as production of the proinflammatory cytokine interleukin (IL)-17A [38]. A role for Collinsella in altering gut permeability and disease severity was confirmed in experimental arthritis. C. aerofaciens can aggravate arthritis in a collagen-induced arthritis model [38]. Collinsella sp could participate in the development of RA through molecular mimicry as well.
C. aerofaciens has been associated with increased ethanol production and liver inflammation. C. aerofaciens has shown an increased abundance in the gut of obese patients living in India [39]. Collinsella aerofaciens was increased in non-constipated irritable bowel syndrome [20]. This bacterium contributes to pro-inflammatory immune states, and is associated with markers of increased endothelial permeability and liver functionality, suggesting an involvement of the gut-liver axis in this condition.
Several bacterial genera, including Prevotella, Bacteroides, Ruminococcus, Blautia, and Collinsella have been reported in association with brain connectivity [40]. The autistic spectrum disorder (ASD)-associated intestinal microbiota exhibits an increased bacterial diversity [41] with enrichment of Collinsella, Corynebacterium, and Lactobacillus.
The associations of C. aerofaciens with pro-inflammatory responses and undesirable outcomes can be leveraged to induce beneficial responses in programmed cell death 1 (PD-1)/programmed death-ligand 1 (PD-L1) cancer immunotherapy [42]. Enrichment of certain commensal such as Bifidobacterium longum, Bifidobacterium adolescentis, Collinsella aerofaciens, and Enterococcus faecium in patients with metastatic melanoma have been associated with anti-PD-1 treatment efficacy [43].
Further research will elucidate the advantages and broaden the applications of these bacteria across a range of conditions.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Author Contributions
VN and JC wrote the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Sharma G, Garg N, Hasan S, Shirodkar S. Prevotella: An insight into its characteristics and associated virulence factors. Microb Pathog. 2022;169:105673.
doi pubmed - Krishnamurthy HK, Pereira M, Bosco J, George J, Jayaraman V, Krishna K, Wang T, et al. Gut commensals and their metabolites in health and disease. Front Microbiol. 2023;14:1244293.
doi pubmed - Sun W, Zhang Y, Guo R, Sha S, Chen C, Ullah H, Zhang Y, et al. A population-scale analysis of 36 gut microbiome studies reveals universal species signatures for common diseases. NPJ Biofilms Microbiomes. 2024;10(1):96.
doi pubmed - Nishijima S, Suda W, Oshima K, Kim SW, Hirose Y, Morita H, Hattori M. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 2016;23(2):125-133.
doi pubmed - Bhute S, Pande P, Shetty SA, Shelar R, Mane S, Kumbhare SV, Gawali A, et al. Molecular characterization and meta-analysis of gut microbial communities illustrate enrichment of Prevotella and Megasphaera in Indian subjects. Front Microbiol. 2016;7:660.
doi pubmed - Nakayama J, Yamamoto A, Palermo-Conde LA, Higashi K, Sonomoto K, Tan J, Lee YK. Impact of westernized diet on gut microbiota in children on Leyte island. Front Microbiol. 2017;8:197.
doi pubmed - De Filippo C, Di Paola M, Ramazzotti M, Albanese D, Pieraccini G, Banci E, Miglietta F, et al. Diet, environments, and gut microbiota. a preliminary investigation in children living in rural and urban Burkina Faso and Italy. Front Microbiol. 2017;8:1979.
doi pubmed - Matsuoka T, Hosomi K, Park J, Goto Y, Nishimura M, Maruyama S, Murakami H, et al. Relationships between barley consumption and gut microbiome characteristics in a healthy Japanese population: a cross-sectional study. BMC Nutr. 2022;8(1):23.
doi pubmed - Lappi J, Salojarvi J, Kolehmainen M, Mykkanen H, Poutanen K, de Vos WM, Salonen A. Intake of whole-grain and fiber-rich rye bread versus refined wheat bread does not differentiate intestinal microbiota composition in Finnish adults with metabolic syndrome. J Nutr. 2013;143(5):648-655.
doi pubmed - Rettedal EA, Altermann E, Roy NC, Dalziel JE. The effects of unfermented and fermented cow and sheep milk on the gut microbiota. Front Microbiol. 2019;10:458.
doi pubmed - Holmes ZC, Villa MM, Durand HK, Jiang S, Dallow EP, Petrone BL, Silverman JD, et al. Microbiota responses to different prebiotics are conserved within individuals and associated with habitual fiber intake. Microbiome. 2022;10(1):114.
doi pubmed - Qin P, Zou Y, Dai Y, Luo G, Zhang X, Xiao L. Characterization a novel butyric acid-producing bacterium Collinsella aerofaciens Subsp. Shenzhenensis Subsp. Nov. Microorganisms. 2019;7(3):78.
doi pubmed - Kordahi MC, DePaolo RW. Commensal bacteria decontaminating your diet. Cell Host Microbe. 2019;26(4):446-448.
doi pubmed - Siegel EL, Lane K, Yuan A, Smalls-Mantey LA, Laird J, Olson C, Hernandez D. Energy insecurity indicators associated with increased odds of respiratory, mental health, and cardiovascular conditions. Health Aff (Millwood). 2024;43(2):260-268.
doi pubmed - Gomez-Arango LF, Barrett HL, Wilkinson SA, Callaway LK, McIntyre HD, Morrison M, Dekker Nitert M. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes. 2018;9(3):189-201.
doi pubmed - Novakovic M, Rout A, Kingsley T, Kirchoff R, Singh A, Verma V, Kant R, et al. Role of gut microbiota in cardiovascular diseases. World J Cardiol. 2020;12(4):110-122.
doi pubmed - Ahmadmehrabi S, Tang WHW. Gut microbiome and its role in cardiovascular diseases. Curr Opin Cardiol. 2017;32(6):761-766.
doi pubmed - Murri M, Leiva I, Gomez-Zumaquero JM, Tinahones FJ, Cardona F, Soriguer F, Queipo-Ortuno MI. Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control study. BMC Med. 2013;11:46.
doi pubmed - Frost F, Storck LJ, Kacprowski T, Gartner S, Ruhlemann M, Bang C, Franke A, et al. A structured weight loss program increases gut microbiota phylogenetic diversity and reduces levels of Collinsella in obese type 2 diabetics: A pilot study. PLoS One. 2019;14(7):e0219489.
doi pubmed - Gargari G, Mantegazza G, Cremon C, Taverniti V, Valenza A, Barbaro MR, Marasco G, et al. Collinsella aerofaciens as a predictive marker of response to probiotic treatment in non-constipated irritable bowel syndrome. Gut Microbes. 2024;16(1):2298246.
doi pubmed - Bassis CM, Erb-Downward JR, Dickson RP, Freeman CM, Schmidt TM, Young VB, Beck JM, et al. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. mBio. 2015;6(2):e00037.
doi pubmed - Bao L, Zhang C, Dong J, Zhao L, Li Y, Sun J. Oral Microbiome and SARS-CoV-2: Beware of Lung Co-infection. Front Microbiol. 2020;11:1840.
doi pubmed - Rodriguez Y, et al. Immunomodulation of human alveolar macrophage response to the SARS-CoV-2 S protein by oral microbiota. Clinical Infection and Immunity. 2024;9(1):16-19.
- Hirayama M, Nishiwaki H, Hamaguchi T, Ito M, Ueyama J, Maeda T, Kashihara K, et al. Intestinal Collinsella may mitigate infection and exacerbation of COVID-19 by producing ursodeoxycholate. PLoS One. 2021;16(11):e0260451.
doi pubmed - Cervantes JR, Barragan J, Dempsey KB, and Palfreeman M. Prevotella differentially regulates the inflammatory response of human monocytes to SARS-CoV-2 spike glycoproteins. J Investig Med. 2021;69(2):1114-1115.
- Utrero-Rico A, Gonzalez-Cuadrado C, Chivite-Lacaba M, Cabrera-Marante O, Laguna-Goya R, Almendro-Vazquez P, Diaz-Pedroche C, et al. Alterations in circulating monocytes predict COVID-19 severity and include chromatin modifications still detectable six months after recovery. Biomedicines. 2021;9(9):1253.
doi pubmed - Ley RE. Gut microbiota in 2015: Prevotella in the gut: choose carefully. Nat Rev Gastroenterol Hepatol. 2016;13(2):69-70.
doi pubmed - Wang L, Wang Y, Zhang P, Song C, Pan F, Li G, Peng L, et al. Gut microbiota changes in patients with spondyloarthritis: A systematic review. Semin Arthritis Rheum. 2022;52:151925.
doi pubmed - Precup G, Vodnar DC. Gut Prevotella as a possible biomarker of diet and its eubiotic versus dysbiotic roles: a comprehensive literature review. Br J Nutr. 2019;122(2):131-140.
doi pubmed - Marietta EV, Murray JA, Luckey DH, Jeraldo PR, Lamba A, Patel R, Luthra HS, et al. Suppression of inflammatory arthritis by human gut-derived prevotella histicola in humanized mice. Arthritis Rheumatol. 2016;68(12):2878-2888.
doi pubmed - Mangalam A, Shahi SK, Luckey D, Karau M, Marietta E, Luo N, Choung RS, et al. Human gut-derived commensal bacteria suppress CNS inflammatory and demyelinating disease. Cell Rep. 2017;20(6):1269-1277.
doi pubmed - Shahi SK, Freedman SN, Murra AC, Zarei K, Sompallae R, Gibson-Corley KN, Karandikar NJ, et al. Prevotella histicola, a human gut commensal, is as potent as COPAXONE(R) in an animal model of multiple sclerosis. Front Immunol. 2019;10:462.
doi pubmed - Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, Rostron T, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202.
doi pubmed - Pianta A, Arvikar S, Strle K, Drouin EE, Wang Q, Costello CE, Steere AC. Evidence of the immune relevance of prevotella copri, a gut microbe, in patients with rheumatoid arthritis. Arthritis Rheumatol. 2017;69(5):964-975.
doi pubmed - Zhang X, Zhang D, Jia H, Feng Q, Wang D, Liang D, Wu X, et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. 2015;21(8):895-905.
doi pubmed - Tsetseri MN, Silman AJ, Keene DJ, Dakin SG. The role of the microbiome in rheumatoid arthritis: a review. Rheumatol Adv Pract. 2023;7(2):rkad034.
doi pubmed - Ruiz-Limon P, Mena-Vazquez N, Moreno-Indias I, Manrique-Arija S, Lisbona-Montanez JM, Cano-Garcia L, Tinahones FJ, et al. Collinsella is associated with cumulative inflammatory burden in an established rheumatoid arthritis cohort. Biomed Pharmacother. 2022;153:113518.
doi pubmed - Chen J, Wright K, Davis JM, Jeraldo P, Marietta EV, Murray J, Nelson H, et al. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016;8(1):43.
doi pubmed - Purohit A, Kandiyal B, Kumar S, Pragasam AK, Kamboj P, Talukdar D, Verma J, et al. Collinsella aerofaciens linked with increased ethanol production and liver inflammation contribute to the pathophysiology of NAFLD. iScience. 2024;27(2):108764.
doi pubmed - Mulder D, Aarts E, Arias Vasquez A, Bloemendaal M. A systematic review exploring the association between the human gut microbiota and brain connectivity in health and disease. Mol Psychiatry. 2023;28(12):5037-5061.
doi pubmed - Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, Youn E, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16(4):444-453.
doi pubmed - Kwon J, Bae M, Szamosvari D, Cassilly CD, Bolze AS, Jackson DR, Xavier RJ, et al. Collinsella aerofaciens Produces a pH-responsive lipid immunogen. J Am Chem Soc. 2023;145(13):7071-7074.
doi pubmed - Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104-108.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Clinical Infection and Immunity is published by Elmer Press Inc.