| Clinical Infection and Immunity, ISSN 2371-4972 print, 2371-4980 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Clin Infect Immun and Elmer Press Inc |
| Journal website https://cii.elmerpub.com |
Review
Volume 10, Number 1, March 2025, pages 1-8
Extracellular DNA-Triggered Inflammation in Arthritis
Drew Johnstona, Anita Lalooa, Jorge Cervantesa, b
aDr. Kiran C. Patel College of Allopathic Medicine, Nova Southeastern University, Fort Lauderdale, FL, USA
bCorresponding Author: Jorge Cervantes, Dr. Kiran C. Patel College of Allopathic Medicine, Nova Southeastern University, Fort Lauderdale, FL, USA
Manuscript submitted January 4, 2025, accepted February 13, 2025, published online February 20, 2025
Short title: Extracellular DNA-Triggered Inflammation in Arthritis
doi: https://doi.org/10.14740/cii503
- Abstract
- Introduction
- Circulating Cell-Free DNA (cfDNA)
- Circulating DNA in Autoimmune Rheumatic Diseases
- Oral Bacterial DNA and Arthritis
- Bacterial DNA Persistence in Brucellosis and Lyme Arthritis
- Bacterial DNA Recognition
- Conclusions
- References
| Abstract | ▴Top |
Cell-free DNA (cfDNA) comprises short fragments of double-stranded DNA, which promotes inflammation through various mechanisms. It can be detected in a variety of diseases, including autoimmune disorders like rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), which has sparked interest for their potential use in diagnosis and therapy. Failure of clearance of extracellular DNA appears to be a major mechanism that may alter tissue homeostasis, leading to inflammation. In this review, we describe the characteristics of cfDNA, the role of bacterial-derived DNA in arthritis, and major sensing mechanisms by toll-like receptor 9 (TLR9). We aim to summarize up-to-date knowledge on the pathogenetic mechanisms of cfDNA in arthritis and discuss recent therapeutic approaches.
Keywords: Extracellular DNA; Circulating cell-free DNA; Extracellular vesicles; Rheumatoid arthritis; SLE; Reactive arthritis; TLR9
| Introduction | ▴Top |
Prokaryotic and eukaryotic cells possess DNA, and this can serve as a ligand of various innate immune receptors including toll-like receptors (TLRs), RIG-I-like receptors (RLRs), and cytosolic DNA sensors [1]. These receptors have been evolutionary preserved from very primitive systems [2]. Sensing of certain forms of DNA can elicit an inflammatory response which can become aberrant in certain individuals. Furthermore, continuous stimulation by persistent DNA in tissue could be involved in the pathogenesis of various reactive and autoimmune conditions. We here focus on the role of free extracellular DNA and its role in inflammatory arthritis.
| Circulating Cell-Free DNA (cfDNA) | ▴Top |
Body fluids such as serum, plasma, urine, cerebrospinal fluid (CSF), saliva, or bronchial effusions have been found to contain circulating cfDNA with increased levels in patients with inflammatory diseases and malignancies. They were initially noted in the 20th century in healthy individuals. There is not much known about the role of cfDNA in autoimmune disease but certainly there has been an increased curiosity in learning more about its role. cfDNA plays a physiological role in immune system regulation, its presence and clearance in healthy individuals is involved in an indispensable homeostatic balance [3, 4].
Composed of degraded fragments of DNA, cfDNA can develop from various sources and causes. The composition of cfDNA is heterogeneous, and it can be bound as a complex of DNA associated with extracellular vesicles (EVs), or as part of larger macromolecular complexes such as nucleosomes. They are also highly variable in length and size (20 - 200 bp) depending on the mechanisms involved in their fragmentation.
Although the origin of cfDNA is currently unknown, there are several postulated mechanisms which describe the origins and sources of cfDNA (Fig. 1). One proposed mechanism is that of active secretion of the cfDNA into the circulation in EVs such as exosomes, microparticles, or apoptotic bodies. These structures protect cfDNA from the action of nucleases to some extent, so cfDNA may be later released upon breakdown of the EVs. It has been proposed that a great majority of cfDNAs are released in this manner. EVs are membrane-bound vesicles released by various cells of our body carrying cell-derived contents such as proteins, lipids, metabolites, and nucleic acids [5]. They play a significant role in intercellular communication in both physiological and pathological conditions [6].
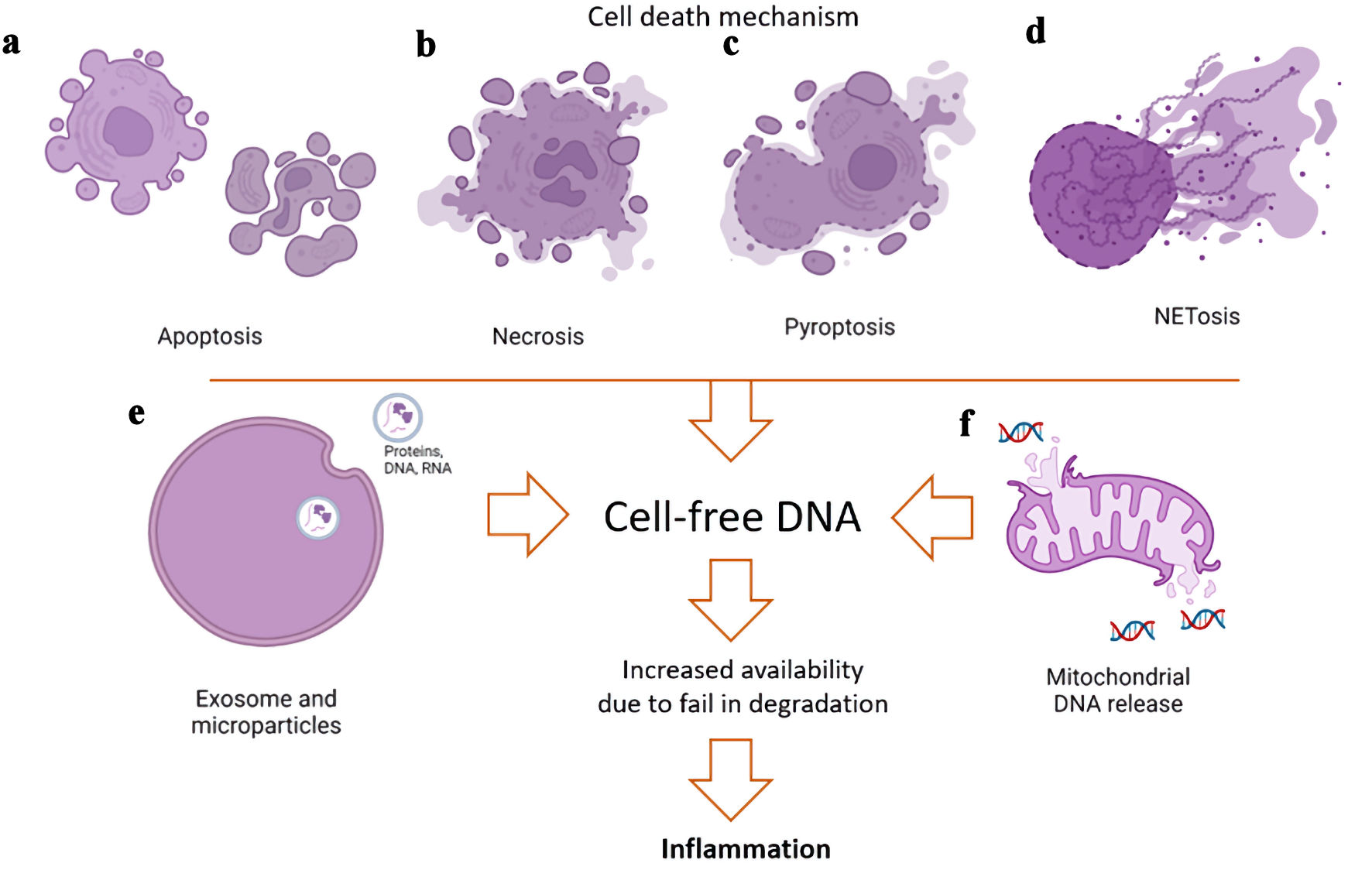 Click for large image | Figure 1. Potential mechanisms of generation of cell-free DNA (cfDNA) in extracellular spaces. The sources of the cfDNA have various etiologies and may be released through normal physiological processes or pathologic states. (a) An important source of extracellular vesicles (EVs) carrying nucleic acids is apoptosis of cells via caspase activation to recycle and degrade components of cells at the end of their life cycle. (b) Conversely, necrosis that occurs in response to injury results in cell swelling and lysis, releasing the cell contents in a rapid and pro-inflammatory manner. (c) Inflammatory insults such as infections can result in pyroptosis, whereby the cell undergoes swelling, membrane blebbing, chromatin condensation, and random DNA fragmentation. (d) Neutrophil extracellular trap (NET) activation and release of DNA allow neutrophils to kill invading pathogens through apoptotic-like changes without activation with caspase. Suicidal NETosis leads to rupturing of the plasma membrane and release of the neutrophil contents into the extracellular space. (e) Exosome or microparticle EV releases with proteins, DNA, or RNA as their cargo. (f) Release of mitochondrial DNA can lead to an increased expression in pro-inflammatory genes by the activation of the cGAS-STING pathway. |
cfDNA can also be released by cell death processes. Apoptosis or programmed cell death is an important component in maintaining cellular homeostasis. Apoptosis uses caspase activation to remove damaged cells as it causes the cell to undergo structural and biochemical changes which include DNA fragmentation. cfDNAs released in this manner are highly fragmented. When there is physical or chemical injury, necrosis leads to a more rapid cell death than apoptosis by cell swelling, which causes a loss of membrane integrity and the release of the cell contents. cfDNA via necrosis is larger in size, as there is no specific chromatin digestion process. cfDNA fragments, similar to necrosis, are seen in NETosis, a type of cell death observed in neutrophils that occurs during both sterile and bacterial inflammation. Neutrophil cell death in NETosis can result in release of inflammatory mitochondrial DNA (mtDNA), a process observed in patients with systemic lupus erythematosus (SLE) [7] and rheumatoid arthritis (RA) [8]. cfDNA during severe systemic inflammation involves hematopoietic cells like neutrophils, but other non-hematopoietic origins exist as well [9].
Another cell death mechanism is that of pyroptosis, an inflammatory process that causes the activation of the inflammasome and caspase-1, leading to the maturation and release of proinflammatory cytokines interleukin-1β (IL-1β) and interleukin-18 (IL-18). This mechanism of cell death occurs rapidly in response to diverse damaging insults such as infections, pathogen- and danger-associated molecular patterns, altered levels of host metabolites, and environmental irritants. Following cell lysis, endogenous host molecules, including DNA, are released into the extracellular space [10].
mtDNA can be released into the cytoplasm and extracellular environments. Intracellular release of mtDNA in the cytoplasm causes increased expression of interferon (IFN)-related and pro-inflammatory genes [11]. Release of mtDNA into the cytoplasm occurs through mitochondrial herniation via membrane pores during apoptosis [12]. This mechanism, however, does not explain which forms of cell-free mtDNA (cf-mtDNA) are released extracellularly. Circulating cf-mtDNA has been identified in various diseases, such as cancer, autoimmune diseases, Parkinson’s disease, Alzheimer’s disease, progressive multiple sclerosis, as well as in stress. It has been thought that factors such as obesity, age, stress and exercise can induce the release of cfDNA into circulation. These are usually very short DNA fragments, more fragmented than the total cfDNA. cf-mtDNA may not correlate with total cfDNA levels, which implies that cf-mtDNA could potentially become an independent biomarker for certain conditions.
The two major DNA-sensing pathways linked to cfDNA are TLR9 recognition, and the stimulator of interferon genes (STING) pathway. Both can be upregulated in inflammatory conditions, augment the uptake of DNA and protect the DNA from degradation. The remaining DNA will then promote the induction of proinflammatory responses.
| Circulating DNA in Autoimmune Rheumatic Diseases | ▴Top |
There are several existing autoimmune diseases which affect 5-10% of the population of the world. Systemic inflammatory autoimmune diseases can be identified by the presence of unique autoantibodies and specific immune cells. Much knowledge has been gained from research in autoimmunity with a focus on identifying new biomarkers, determining the mechanisms related to the pathogenesis of the autoimmune disease and creating new therapeutic agents. In 1966, high values of cfDNA were identified in patients with SLE [13, 14]. Since then, the role of nucleic acid in the pathogenesis of autoimmune diseases has been a key area of focus in the area of research. The ability to identify and evaluate cfDNA shows great potential as a biomarker for disease activity, progression and possible therapeutic modality in autoimmune diseases.
RA is probably the most common chronic autoimmune arthritis [15]. Although remission is possible, outcomes as well as treatment modalities vary considerably. Although there are biomarkers used in the early diagnosis of RA such as anticitrullinated protein antibodies (ACPA) and rheumatoid factor (RF), more information is sought regarding novel biomarkers to be used in the diagnosis, categorization and to help predict treatment responsiveness. The analysis of cfDNA, and how they can guide individualized treatment is an avenue of huge potential interest [16]. cfDNA in patients with RA is elevated in peripheral blood and synovial fluid and is associated with disease activity [10, 17]. Reduced levels of serum cfDNA in established RA compared to healthy controls, and early RA patients [18], suggest that their role may be key in the initiation of the disease. This may help guide the optimal time for newer treatments utilizing DNase-conjugated nanogels for active cfDNA degradation in the management of RA [19].
cfDNA and extracellular mtDNA in synovial fluid show that cfDNA is primarily located in the joints of RA patients [20], where it induces upregulation of cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) [21] which lead to RA progression [22]. Release of cfDNA could increase in conditions including trauma, infections, or other mechanisms. Synovial fluid cfDNA is full of specific hypomethylated CpG motif-rich sequences known to be proinflammatory in RA-related cells [23]. cfDNA and mtDNA could also possibly bind with antimicrobial peptides or the high mobility group box chromosomal protein 1 (HMGB1), a non-histone protein from activated and dying cells involved in autoimmunity [22].
It has been shown that cfDNA could forecast the effects of treatment with biological disease-modifying anti-rheumatic drugs (bDMARDs) in RA patients, as there was an increase in cfDNA 8 weeks after starting bDMARDs [24]. Other studies, however, showed decreases in plasma cfDNA during treatment with dDMARDs [25]. This demonstrates the necessity for further research in analyzing cfDNA in RA patients on treatment. Nevertheless, this opens the door for the identification of a possible biomarker for RA, and how it could add diagnostic or prognostic value to ACPA and RF [26].
Arthritis in SLE is common and has a significant impact on patients in terms of their burden of disease and quality of life [27]. Endogenous or self-DNA has emerged as a potent trigger mechanism in SLE, with self-reactive B cells supposedly to be edited to prevent autoimmunity, playing an important role. Self-reactive B-cell receptors are present in 10-20% of all mature naive B cells in healthy individuals, this percentage increases up to 50% in SLE, with significant self-DNA reactivity correlating with disease severity [28].
Increased availability of immunogenic self-DNA, as an immunogen or adjuvant, may be the initiating factor for the loss of tolerance causing production of anti-DNA antibodies by self-DNA-reactive B cells and subsequent manifestations of SLE [28]. The sources of DNA in SLE are multiple, and the heterogeneity, in which pathogenic nucleic acid molecules are present and which sensors and pathways they trigger, contributes to the clinical heterogeneity of SLE [29]. Mutations impairing apoptotic cell clearance pathways and nucleic acid metabolism-associated genes like DNases, are known to play a role in this increased availability of DNA [30, 31] (Fig. 2). Autoimmune diseases characterized by pathogenic DNA accumulation, such as SLE, can be effectively treated with a replacement DNAse to bypass pathogenic mechanisms [32].
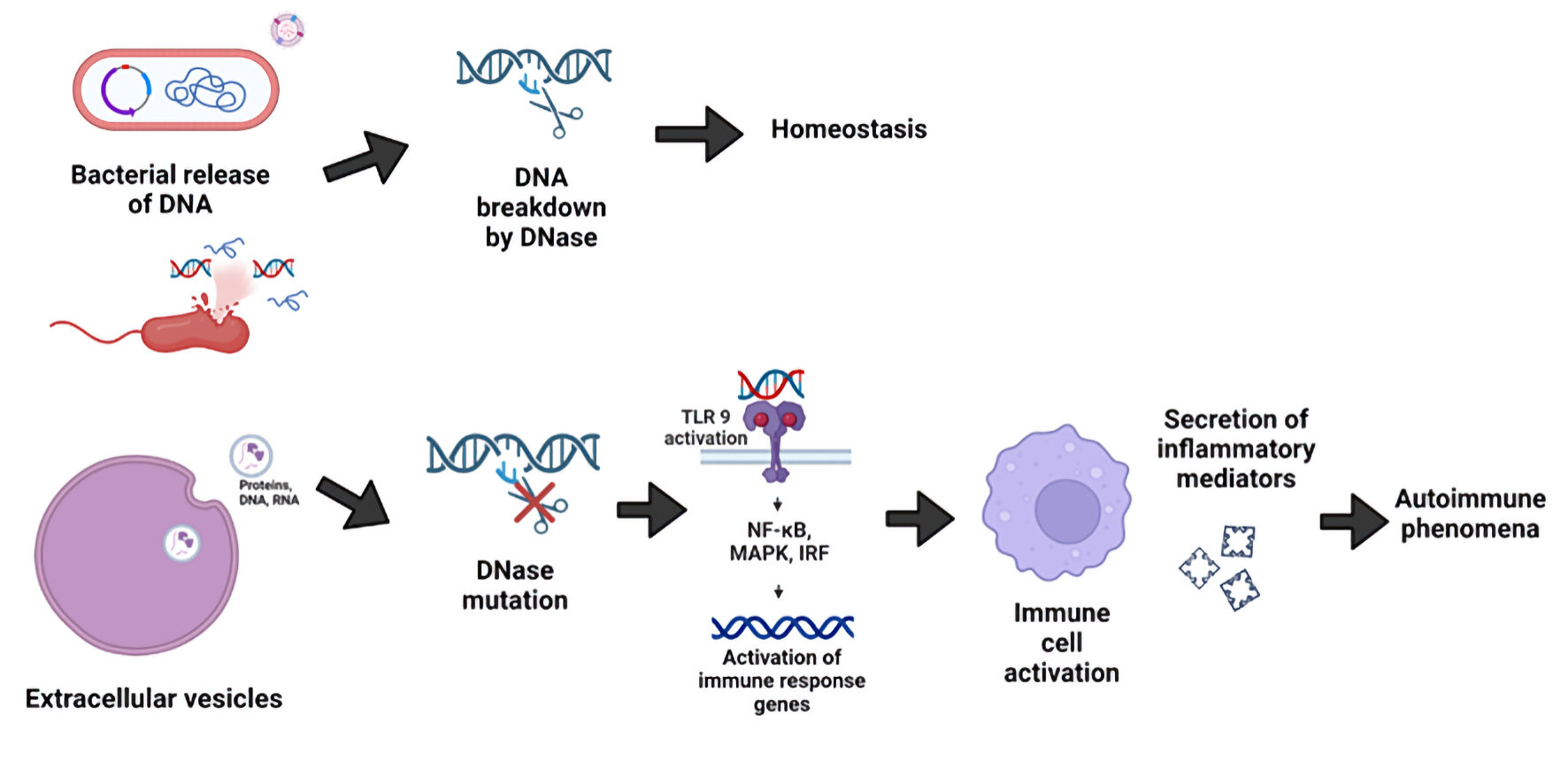 Click for large image | Figure 2. Induction of autoimmune phenomena by cell-free DNA (cfDNA) upon its recognition by toll-like receptor 9 (TLR9). DNases are important in the reduction of inflammatory responses and in the maintenance of homeostasis. Self and foreign (bacterial)-derived cfDNA in human tissues may persist when nucleic acids fail to be degraded by DNases. This can lead to TLR9 activation in immune cells, with an ensuing inflammatory response and induction of autoimmunity. |
There is very little information on the importance of cfDNA in other autoimmune spondyloarthropathies such as ankylosing spondylitis (AS), psoriatic arthritis (PsA) or reactive arthritis (ReA). There is no clear consensus of the levels of DNA in serum and synovial fluid from patients with arthritis, including psoriatic arthropathy and AS (Leon et al, 1981 [20]). Synovial fluid from SpA patients has shown that cfDNA correlates with proteins in neutrophil granulocytes [33]. Future research in this area is needed to further investigate the role of cfDNA in these autoimmune conditions.
| Oral Bacterial DNA and Arthritis | ▴Top |
Although RA is considered mainly an autoimmune disorder, RA-associated human leukocyte antigens shape microbiomes and increase the risk of dysbiosis in mucosae. RA might also be induced by epigenetic changes in long-lived synovial presenting cells, which are stressed by excessive translocation into joints of bacteria from the poorly cultivable gut, lung, or oral microbiota, in the same way as more pathogenic bacteria can lead to “reactive arthritis” [34]. Bacterial composition can even be used as noninvasive biomarkers for arthritis screening [35], being able to differentiate RA from osteoarthritis (OA).
Not only can oral microbial dysbiosis increase susceptibility to develop RA [36], but several studies have demonstrated the association between periodontitis with severity and progression of RA [37]. The number of joints with movement restrictions caused by RA correlates with the number of missing teeth in these patients [38]. Furthermore, RA patients may be able to experience rapid and dramatic improvement by surgical treatment of unresolved oral infections [39]. This is supportive of the temporal association between oral infections and chronic systemic autoimmune disease such as RA. In fact, development and novel therapeutic interventions in RA are focusing on oral infections [40].
ReA is an inflammatory arthritis that presents days or weeks after a gastrointestinal or genitourinary infection. The Reiter’s syndrome, named after Hans Reiter, is described as a classic triad of arthritis, urethritis, and conjunctivitis, although the majority of patients do not present with the classic triad. It is currently believed that the disorder is due to an aberrant autoimmune following a gastrointestinal or genitourinary infection caused by Salmonella, Shigella, Campylobacter, or Chlamydia [41]. It can also present after a dental infection occurring after a dental extraction [39].
The presence of periodontal pathogens DNA can be found in synovial fluid, which strongly suggests that oral bacteria may play a role in the pathogenesis of arthritis. In patients with RA and OA, DNA of Porphyromonas gingivalis (P. gingivalis) can be detected in synovial fluid more often than in controls. DNA from P. gingivalis was present in both oral plaque and synovial fluid [38, 42]. The oral-synovium translocation could be due to the periodontal diseases of the patients [43].
The oral bacterial DNA found in synovial fluids of arthritis patients seems to be of different species between arthritides. While Actinobacillus naeslundii, Streptococcus constellatus, Eubacterium nodatum, P. gingivalis, Actinomyces viscosus, Treponema denticola, Prevotella nigrescens, and Neisseria mucosa were pretty much present in all arthritides, Streptococcus anginosus, and Actinomyces israelii, were higher in RA, and along with various species like Eubacterium saburreum, Actinobacillus actinomycetemcomitans, Streptococcus intermedius, Tannerella forsythensis, Prevotella melaninogenica and Prevotella intermedia were completely absent in controls [42]. This strongly indicates a possible connection between oral infections and joint inflammation [42].
| Bacterial DNA Persistence in Brucellosis and Lyme Arthritis | ▴Top |
Brucellosis is a chronic infectious disease with multiple inflammatory manifestations. Brucella is a microorganism that shows preferential tissue tropism to certain organs sites such as the reproductive system, bone, and fetal ectoderm. Early manifestations of brucellosis consist of fever, sweating, joint pain, symptoms of poisoning, chronic spine arthritis, orchitis, ovarian inflammation, and neurological manifestations. More bacteria are released into the blood upon replication in organs and tissues, which increases the risk of complications in brucellosis [44]. Osteoarticular features, such as arthritis, occur in around 14-26% of the patients suffering from acute, sub-acute or chronic brucellosis [45], making it easily mistaken for RA [46].
After acute brucellosis infection, symptoms persist in a minority of patients for more than 1 year. Such patients are defined as having chronic brucellosis. DNA persistence is detected in all focal-disease patients and symptomatic nonfocal-disease patients with chronic brucellosis [47]. This study analyzed Brucella DNA persistence in the serum from brucellosis patients, most of whom manifested polyarthralgia. Curiously, the increase in DNA was observed in serum and not in blood in follow-up. We were unable to find any study regarding Brucella DNA persistence in joints of patients with chronic brucellosis. This is still a possibility given that Brucella DNA is present in the synovial fluid of acute brucellosis arthritis [48], and DNA amplification on joint fluids is useful when culture results have proved inconclusive [49].
A longitudinal study showed that the majority of brucellosis patients exhibit persistent detectable bacterial DNA load despite being asymptomatic, with 70% even more than 2 years after treatment [50]. This is even more curious, given that some of these patients were culture negative for Brucella. The isolation rate of the fastidious etiologic agent from blood cultures is low, and therefore laboratory diagnosis is mainly based on serologic and molecular testing. However, seronegative brucellosis patients have been described, and antibody titers of diagnostic significance are difficult to define [51].
Lyme disease is a zoonosis caused by infection with the spirochete Borrelia burgdorferi (B. burgdorferi). The disease is inflammatory in nature, as the spirochete is not well adapted to humans. Articular joints are a major target of B. burgdorferi, with the occurrence of Lyme arthritis differing between geographical regions due to the presence of various species of B. burgdorferi or the immunogenetic background of the host [52]. In some individuals, chronic inflammatory symptomatology persists despite antibiotic therapy. One of the possible explanations for such phenomenon is that B. burgdorferi DNA can persist for long periods of time in some individuals, even after antibiotic therapy. Evidence suggests that after antibiotic eradication of B. burgdorferi, its DNA is able to persist in anatomical locations that coincide with sites of inflammation [53, 54]. cfDNA can be detected in 64% with laboratory-confirmed Lyme disease at an early stage [55], which proposes a potential diagnostic utility of a disease that is sometimes difficult to diagnose.
In other conditions like the mouse model of chronic colitis used as a surrogate for inflammatory bowel disease (IBD), bacterial DNA derived from luminal bacteria contributes significantly to the perpetuation of chronic intestinal inflammation. This is greatly dependent on TLR9 [56]. Even more interesting is the fact that TLR9 plays a major role in the T-cell-dependent phases of inflammatory erosive autoimmune arthritis [57]. Unmethylated CpG motifs from intra-articularly localized bacterial DNA can induce arthritis by activating macrophages [58].
| Bacterial DNA Recognition | ▴Top |
Endosomal TLRs, i.e., TLRs 3, 7, 8, and 9 recognize self and foreign double-stranded RNA and single-stranded RNA and DNA [59]. The development of therapeutics to inhibit the endosomal TLRs or components of their signaling cascades may represent a way to target inflammation upstream of cytokine production [60].
TLR9 is the best characterized sensor for bacterial DNA (Fig. 2), which contains short sequences of unmethylated CpG motifs, though TLR9-independent intracellular DNA recognition mechanisms may also exist [61]. Certain immune cells like dendritic cells, B cells, and neutrophils show considerable expression of TLR9 [62, 63]. Neutrophils contribute in great extent to the tissue damage in many inflammatory diseases, including RA [61]. When neutrophil recruitment into the joint is prevented, through natural killer (NK) cell-derived IFN-γ, inhibition of arthritis development is observed [64].
Although TLR9 is active in the endosomal compartment for DNA recognition in dendritic cells, and B cells [1], neutrophils are able to bind pathogen-derived unmethylated CpG motifs to surface TLR9 [63]. This pathway may be triggered when pathogen-derived TLR9 ligands cannot reach the endosome, offering a rescue mechanism for neutrophil activation.
TLR9-mediated recognition of the unmethylated CpG motifs can lead to distinct signaling responses depending on the sequence of oligodeoxynucleotide (ODN)s that express CpG motifs [65]. ODN1688 can exacerbate inflammation in the colon but blocks arthritis development in certain murine models [64], whereas CpG-DNA has been reported to promote arthritis in other model systems. Since these studies did not always use the same CpG-ODNs, interpretation remains a complex challenge.
DNA present in the extracellular space, leftover from damaged microbes or infected host cells, may be degraded by extracellular DNases present in that site. Such degradation appears to be important in maintaining a homeostatic balance. When a mutation or a deficiency occurs in one of these DNases, the presence of this remaining DNA nucleic acid can trigger an autoimmune response, like lupus and lupus arthritis [30].
Oral microbial extracellular DNA can initiate periodontitis through gingival degradation by fibroblast-derived mechanisms [66]. Oral tolerance induced by bacterial DNA-mediated expansion of Breg cells suppress disease onset in an autoimmune-prone mouse model. For SLE, gut microbiota and bacterial DNA suppress autoimmunity by stimulating regulatory B cells in a murine model of lupus [67].
Periodontitis and RA are both chronic inflammatory diseases, and they share immunological and inflammatory mechanisms, causing soft tissue inflammation and bone destruction. RA can promote periodontitis-associated bone destruction in the lesion area. New potential therapeutic strategies to reduce macrophage infiltration, TLR9, and the increase of inflammatory cytokines in the periodontitis, are being pursued. The goal is to suppress the activation of TLR9 by CpG ODN, and thus the TLR9 downstream signaling involved in inflammation [68]. Both gram-positive and gram-negative bacteria can produce EVs in the form of outer membrane vesicles (OMVs), used for communication with host cells and other bacteria. OMV cargo includes RNA, DNA, proteins, and virulence factors [69]. Multiple studies have found that OMVs participate in various inflammatory diseases, including periodontitis, gastrointestinal and pulmonary inflammation, and sepsis. OMVs can trigger pattern recognition receptors, activating inflammasomes and inducing mitochondrial dysfunction. OMVs can also affect inflammation in distant organs or tissues via long-distance cargo transport [70]. The OMVs produced by P. gingivalis may be involved in the progression of periodontitis [71]. Fusobacterium nucleatum (F. nucleatum), another oral and gastrointestinal organism, is enriched in RA patients and positively associated with RA severity. F. nucleatum OMVs containing the virulence determinants translocate into the joints, triggering local inflammatory responses [72].
| Conclusions | ▴Top |
Our review indicates that the presence of DNA in certain individuals could play a major role in the inflammatory phenomena associated with autoimmune arthritis, as well as arthritis associated with certain infectious diseases.
Various lines of research point out that failure to remove DNA in tissues elicits TLR9 recognition and signaling pathways, which could act as possible major mechanisms of excessive inflammation. Although this hypothesis is not fully demonstrated for certain conditions such as arthritis in chronic brucellosis, further research may be able to identify unique mechanisms for this particular infection. Recent literature is suggestive of the role of commensal-derived DNA as aggravating certain inflammatory conditions. Future research should not only clarify but expand our knowledge of DNA-incited joint inflammation.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Author Contributions
JC contributed to the conceptualization. DJ, AL, and JC wrote the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Kawasaki T, Kawai T, Akira S. Recognition of nucleic acids by pattern-recognition receptors and its relevance in autoimmunity. Immunol Rev. 2011;243(1):61-73.
doi pubmed - Martins YC, Ribeiro-Gomes FL, Daniel-Ribeiro CT. A short history of innate immunity. Mem Inst Oswaldo Cruz. 2023;118:e230023.
doi pubmed - Andargie TE, Roznik K, Redekar N, Hill T, Zhou W, Apalara Z, Kong H, et al. Cell-free DNA reveals distinct pathology of multisystem inflammatory syndrome in children. J Clin Invest. 2023;133(21):e171729.
doi pubmed - Korabecna M, Zinkova A, Brynychova I, Chylikova B, Prikryl P, Sedova L, Neuzil P, et al. Cell-free DNA in plasma as an essential immune system regulator. Sci Rep. 2020;10(1):17478.
doi pubmed - Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373-383.
doi pubmed - Thakur K, Singh MS, Feldstein-Davydova S, Hannes V, Hershkovitz D, Tsuriel S. Extracellular vesicle-derived DNA vs. CfDNA as a biomarker for the detection of colon cancer. Genes (Basel). 2021;12(8):1171.
doi pubmed - Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, Malech HL, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med. 2016;22(2):146-153.
doi pubmed - Sur Chowdhury C, Giaglis S, Walker UA, Buser A, Hahn S, Hasler P. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res Ther. 2014;16(3):R122.
doi pubmed - van der Meer AJ, Kroeze A, Hoogendijk AJ, Soussan AA, Ellen van der Schoot C, Wuillemin WA, Voermans C, et al. Systemic inflammation induces release of cell-free DNA from hematopoietic and parenchymal cells in mice and humans. Blood Adv. 2019;3(5):724-728.
doi pubmed - Hashimoto T, Yoshida K, Hashiramoto A, Matsui K. Cell-free DNA in rheumatoid arthritis. Int J Mol Sci. 2021;22(16):8941.
doi pubmed - Trumpff C, Michelson J, Lagranha CJ, Taleon V, Karan KR, Sturm G, Lindqvist D, et al. Stress and circulating cell-free mitochondrial DNA: A systematic review of human studies, physiological considerations, and technical recommendations. Mitochondrion. 2021;59:225-245.
doi pubmed - McArthur K, Whitehead LW, Heddleston JM, Li L, Padman BS, Oorschot V, Geoghegan ND, et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science. 2018;359(6378):eaao6047.
doi pubmed - Tan EM, Kunkel HG. Characteristics of a soluble nuclear antigen precipitating with sera of patients with systemic lupus erythematosus. J Immunol. 1966;96(3):464-471.
pubmed - Tan EM, Schur PH, Carr RI, Kunkel HG. Deoxybonucleic acid (DNA) and antibodies to DNA in the serum of patients with systemic lupus erythematosus. J Clin Invest. 1966;45(11):1732-1740.
doi pubmed - Tobon GJ, Youinou P, Saraux A. The environment, geo-epidemiology, and autoimmune disease: Rheumatoid arthritis. J Autoimmun. 2010;35(1):10-14.
doi pubmed - Coras R, Narasimhan R, Guma M. Liquid biopsies to guide therapeutic decisions in rheumatoid arthritis. Transl Res. 2018;201:1-12.
doi pubmed - Zhong XY, von Muhlenen I, Li Y, Kang A, Gupta AK, Tyndall A, Holzgreve W, et al. Increased concentrations of antibody-bound circulatory cell-free DNA in rheumatoid arthritis. Clin Chem. 2007;53(9):1609-1614.
doi pubmed - Dunaeva M, Buddingh BC, Toes RE, Luime JJ, Lubberts E, Pruijn GJ. Decreased serum cell-free DNA levels in rheumatoid arthritis. Auto Immun Highlights. 2015;6(1-2):23-30.
doi pubmed - Zhu H, Kong B, Che J, Zhao Y, Sun L. Bioinspired nanogels as cell-free DNA trapping and scavenging organelles for rheumatoid arthritis treatment. Proc Natl Acad Sci U S A. 2023;120(33):e2303385120.
doi pubmed - Leon SA, Revach M, Ehrlich GE, Adler R, Petersen V, Shapiro B. DNA in synovial fluid and the circulation of patients with arthritis. Arthritis Rheum. 1981;24(9):1142-1150.
doi pubmed - Dong C, Liu Y, Sun C, Liang H, Dai L, Shen J, Wei S, et al. Identification of specific joint-inflammatogenic cell-free DNA molecules from synovial fluids of patients with rheumatoid arthritis. Front Immunol. 2020;11:662.
doi pubmed - Mondelo-Macia P, Castro-Santos P, Castillo-Garcia A, Muinelo-Romay L, Diaz-Pena R. Circulating Free DNA and Its Emerging Role in Autoimmune Diseases. J Pers Med. 2021;11(2):151.
doi pubmed - Liu Y, Aryee MJ, Padyukov L, Fallin MD, Hesselberg E, Runarsson A, Reinius L, et al. Epigenome-wide association data implicate DNA methylation as an intermediary of genetic risk in rheumatoid arthritis. Nat Biotechnol. 2013;31(2):142-147.
doi pubmed - Hashimoto T, Yoshida K, Hashimoto N, Nakai A, Kaneshiro K, Suzuki K, Kawasaki Y, et al. Circulating cell free DNA: a marker to predict the therapeutic response for biological DMARDs in rheumatoid arthritis. Int J Rheum Dis. 2017;20(6):722-730.
doi pubmed - Laukova L, Konecna B, Vlkova B, Mlynarikova V, Celec P, Stenova E. Anti-cytokine therapy and plasma DNA in patients with rheumatoid arthritis. Rheumatol Int. 2018;38(8):1449-1454.
doi pubmed - Rykova E, Sizikov A, Roggenbuck D, Antonenko O, Bryzgalov L, Morozkin E, Skvortsova K, et al. Circulating DNA in rheumatoid arthritis: pathological changes and association with clinically used serological markers. Arthritis Res Ther. 2017;19(1):85.
doi pubmed - Ball EM, Bell AL. Lupus arthritis—do we have a clinically useful classification? Rheumatology (Oxford). 2012;51(5):771-779.
doi pubmed - Soni C, Reizis B. Self-DNA at the epicenter of SLE: immunogenic forms, regulation, and effects. Front Immunol. 2019;10:1601.
doi pubmed - Mustelin T, Lood C, Giltiay NV. Sources of pathogenic nucleic acids in systemic lupus erythematosus. Front Immunol. 2019;10:1028.
doi pubmed - Napirei M, Karsunky H, Zevnik B, Stephan H, Mannherz HG, Moroy T. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat Genet. 2000;25(2):177-181.
doi pubmed - Hartl J, Serpas L, Wang Y, Rashidfarrokhi A, Perez OA, Sally B, Sisirak V, et al. Autoantibody-mediated impairment of DNASE1L3 activity in sporadic systemic lupus erythematosus. J Exp Med. 2021;218(5):e20201138.
doi pubmed - Stabach PR, Sims D, Gomez-Banuelos E, Zehentmeier S, Dammen-Brower K, Bernhisel A, Kujawski S, et al. A dual-acting DNASE1/DNASE1L3 biologic prevents autoimmunity and death in genetic and induced lupus models. JCI Insight. 2024;9(14):e177003.
doi pubmed - Birkelund S, Bennike TB, Kastaniegaard K, Lausen M, Poulsen TBG, Kragstrup TW, Deleuran BW, et al. Proteomic analysis of synovial fluid from rheumatic arthritis and spondyloarthritis patients. Clin Proteomics. 2020;17:29.
doi pubmed - Berthelot JM, Bandiaky ON, Le Goff B, Amador G, Chaux AG, Soueidan A, Denis F. Another Look at the Contribution of oral microbiota to the pathogenesis of rheumatoid arthritis: a narrative review. Microorganisms. 2021;10(1):59.
doi pubmed - Chen B, Zhao Y, Li S, Yang L, Wang H, Wang T, Bin S, et al. Variations in oral microbiome profiles in rheumatoid arthritis and osteoarthritis with potential biomarkers for arthritis screening. Sci Rep. 2018;8(1):17126.
doi pubmed - Tong Y, Zheng L, Qing P, Zhao H, Li Y, Su L, Zhang Q, et al. Oral microbiota perturbations are linked to high risk for rheumatoid arthritis. Front Cell Infect Microbiol. 2019;9:475.
doi pubmed - Krutyholowa A, Strzelec K, Dziedzic A, Bereta GP, Lazarz-Bartyzel K, Potempa J, Gawron K. Host and bacterial factors linking periodontitis and rheumatoid arthritis. Front Immunol. 2022;13:980805.
doi pubmed - Reichert S, Haffner M, Keysser G, Schafer C, Stein JM, Schaller HG, Wienke A, et al. Detection of oral bacterial DNA in synovial fluid. J Clin Periodontol. 2013;40(6):591-598.
doi pubmed - Maytin L, Morrison J. Reactive Arthritis Resulting From Postoperative Complications of Third Molar Extraction: A Case Report. Cureus. 2022;14(8):e28325.
doi pubmed - Afrasiabi S, Chiniforush N, Partoazar A, Goudarzi R. The role of bacterial infections in rheumatoid arthritis development and novel therapeutic interventions: Focus on oral infections. J Clin Lab Anal. 2023;37(8):e24897.
doi pubmed - Cheeti A, Chakraborty RK, Ramphul K. Reactive arthritis. In: StatPearls. Treasure Island (FL) ineligible companies. 2025.
pubmed - Moen K, Brun JG, Valen M, Skartveit L, Eribe EK, Olsen I, Jonsson R. Synovial inflammation in active rheumatoid arthritis and psoriatic arthritis facilitates trapping of a variety of oral bacterial DNAs. Clin Exp Rheumatol. 2006;24(6):656-663.
pubmed - Temoin S, Chakaki A, Askari A, El-Halaby A, Fitzgerald S, Marcus RE, Han YW, et al. Identification of oral bacterial DNA in synovial fluid of patients with arthritis with native and failed prosthetic joints. J Clin Rheumatol. 2012;18(3):117-121.
doi pubmed - Che LH, Qi C, Bao WG, Ji XF, Liu J, Du N, Gao L, et al. Monitoring the course of Brucella infection with qPCR-based detection. Int J Infect Dis. 2019;89:66-71.
doi pubmed - Esmaeilnejad-Ganji SM, Esmaeilnejad-Ganji SMR. Osteoarticular manifestations of human brucellosis: A review. World J Orthop. 2019;10(2):54-62.
doi pubmed - Sharma V, Sharma A. Infectious mimics of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2022;36(1):101736.
doi pubmed - Castano MJ, Solera J. Chronic brucellosis and persistence of Brucella melitensis DNA. J Clin Microbiol. 2009;47(7):2084-2089.
doi pubmed - Wang J, Zhang Q. Early diagnosis and treatment of acute brucellosis knee arthritis complicated by acute osteomyelitis: two cases report. BMC Infect Dis. 2022;22(1):430.
doi pubmed - Halstead F, Tennant E, Wordworth B. I could smell there was something wrong with him: Clinical case of a decade of chronic undiagnosed brucellosis. Clinical Infection in Practice. 2024. p. 24.
- Vrioni G, Pappas G, Priavali E, Gartzonika C, Levidiotou S. An eternal microbe: Brucella DNA load persists for years after clinical cure. Clin Infect Dis. 2008;46(12):e131-136.
doi pubmed - Al Dahouk S, Nockler K. Implications of laboratory diagnosis on brucellosis therapy. Expert Rev Anti Infect Ther. 2011;9(7):833-845.
doi pubmed - Brouwer MAE, van de Schoor FR, Vrijmoeth HD, Netea MG, Joosten LAB. A joint effort: The interplay between the innate and the adaptive immune system in Lyme arthritis. Immunol Rev. 2020;294(1):63-79.
doi pubmed - Cervantes J. Doctor says you are cured, but you still feel the pain. Borrelia DNA persistence in Lyme disease. Microbes Infect. 2017;19(9-10):459-463.
doi pubmed - Marks CM, Nawn JE, Caplow JA. Gene sequencing and cases of chronic lyme disease-reply. JAMA Intern Med. 2017;177(5):739-740.
doi pubmed - Branda JA, Lemieux JE, Blair L, Ahmed AA, Hong DK, Bercovici S, Blauwkamp TA, et al. Detection of borrelia burgdorferi cell-free DNA in human plasma samples for improved diagnosis of early lyme borreliosis. Clin Infect Dis. 2021;73(7):e2355-e2361.
doi pubmed - Obermeier F, Dunger N, Strauch UG, Hofmann C, Bleich A, Grunwald N, Hedrich HJ, et al. CpG motifs of bacterial DNA essentially contribute to the perpetuation of chronic intestinal inflammation. Gastroenterology. 2005;129(3):913-927.
doi pubmed - Fischer A, Abdollahi-Roodsaz S, Bohm C, Niederreiter B, Meyer B, Yau ACY, Lonnblom E, et al. The involvement of Toll-like receptor 9 in the pathogenesis of erosive autoimmune arthritis. J Cell Mol Med. 2018;22(9):4399-4409.
doi pubmed - Deng GM, Nilsson IM, Verdrengh M, Collins LV, Tarkowski A. Intra-articularly localized bacterial DNA containing CpG motifs induces arthritis. Nat Med. 1999;5(6):702-705.
doi pubmed - Trivedi S, Greidinger EL. Endosomal Toll-like receptors in autoimmunity: mechanisms for clinical diversity. Therapy. 2009;6(3):433-442.
doi pubmed - Thwaites R, Chamberlain G, Sacre S. Emerging role of endosomal toll-like receptors in rheumatoid arthritis. Front Immunol. 2014;5:1.
doi pubmed - El Kebir D, Jozsef L, Filep JG. Neutrophil recognition of bacterial DNA and Toll-like receptor 9-dependent and -independent regulation of neutrophil function. Arch Immunol Ther Exp (Warsz). 2008;56(1):41-53.
doi pubmed - Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, et al. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J Immunol. 2002;168(9):4531-4537.
doi pubmed - Lindau D, Mussard J, Wagner BJ, Ribon M, Ronnefarth VM, Quettier M, Jelcic I, et al. Primary blood neutrophils express a functional cell surface Toll-like receptor 9. Eur J Immunol. 2013;43(8):2101-2113.
doi pubmed - Wu HJ, Sawaya H, Binstadt B, Brickelmaier M, Blasius A, Gorelik L, Mahmood U, et al. Inflammatory arthritis can be reined in by CpG-induced DC-NK cell cross talk. J Exp Med. 2007;204(8):1911-1922.
doi pubmed - Steinhagen F, Meyer C, Tross D, Gursel M, Maeda T, Klaschik S, Klinman DM. Activation of type I interferon-dependent genes characterizes the "core response" induced by CpG DNA. J Leukoc Biol. 2012;92(4):775-785.
doi pubmed - Kondo T, Okawa H, Hokugo A, Shokeen B, Sundberg O, Zheng Y, McKenna CE, et al. Oral microbial extracellular DNA initiates periodontitis through gingival degradation by fibroblast-derived cathepsin K in mice. Commun Biol. 2022;5(1):962.
doi pubmed - Mu Q, Edwards MR, Swartwout BK, Cabana Puig X, Mao J, Zhu J, Grieco J, et al. Gut microbiota and bacterial DNA suppress autoimmunity by stimulating regulatory B cells in a murine model of lupus. Front Immunol. 2020;11:593353.
doi pubmed - Wei W, Ren J, Yin W, Ding H, Lu Q, Tan L, Deng S, et al. Inhibition of Ctsk modulates periodontitis with arthritis via downregulation of TLR9 and autophagy. Cell Prolif. 2020;53(1):e12722.
doi pubmed - Hosseini-Giv N, Basas A, Hicks C, El-Omar E, El-Assaad F, Hosseini-Beheshti E. Bacterial extracellular vesicles and their novel therapeutic applications in health and cancer. Front Cell Infect Microbiol. 2022;12:962216.
doi pubmed - Chen S, Lei Q, Zou X, Ma D. The role and mechanisms of gram-negative bacterial outer membrane vesicles in inflammatory diseases. Front Immunol. 2023;14:1157813.
doi pubmed - Fan R, Zhou Y, Chen X, Zhong X, He F, Peng W, Li L, et al. Porphyromonas gingivalis Outer Membrane Vesicles Promote Apoptosis via msRNA-Regulated DNA Methylation in Periodontitis. Microbiol Spectr. 2023;11(1):e0328822.
doi pubmed - Hong M, Li Z, Liu H, Zheng S, Zhang F, Zhu J, Shi H, et al. Fusobacterium nucleatum aggravates rheumatoid arthritis through FadA-containing outer membrane vesicles. Cell Host Microbe. 2023;31(5):798-810.e797.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Clinical Infection and Immunity is published by Elmer Press Inc.