| Clinical Infection and Immunity, ISSN 2371-4972 print, 2371-4980 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, Clin Infect Immun and Elmer Press Inc |
| Journal website https://cii.elmerpub.com |
Case Report
Volume 000, Number 000, April 2025, pages 000-000
A Case of Severe Malaria With Disseminated Intravascular Coagulation in an American Missionary and Review of Current Treatment and Prophylaxis
Alec Lippmanna, c, Shayan Samavatia, Muhammad Awana, Ryan Yanga, Roshan Dinparastisalehb
aAlabama College of Osteopathic Medicine, Dothan, AL 36303, USA
bDepartment of Internal Medicine, North Alabama Medical Center, Florence, AL 35630, USA
cCorresponding Author: Alec Lippmann, Alabama College of Osteopathic Medicine, Dothan, AL 36303, USA
Manuscript submitted March 21, 2025, accepted March 27, 2025, published online April 7, 2025
Short title: A Case of Severe Malaria in a Missionary
doi: https://doi.org/10.14740/cii505
| Abstract | ▴Top |
Malaria remains a significant global health concern, particularly in endemic regions. Despite the availability of preventive measures, cases continue to emerge in the United States of America (USA), especially among travelers such as missionaries, military personnel, and aid workers to endemic areas. Factors contributing to morbidity and mortality include suboptimal prophylactic protocols, delayed diagnosis and treatment, and the limited availability of life-saving antimalarial medications like artesunate. This case report examines the clinical course and challenges faced by a 47-year-old male American missionary who developed severe malaria after returning from Nigeria. Initially, the patient was misdiagnosed with sepsis due to his nonspecific symptoms, which included fever, weakness and hematuria. However, a peripheral blood smear later confirmed he was infected with malaria. Despite receiving oral antimalarial therapy, the patient’s condition worsened, leading to disseminated intravascular coagulation (DIC) and severe malaria. Artesunate, the recommended treatment for severe malaria, was not immediately available at our hospital, prompting the urgent transportation of the medication from a tertiary care center. After receiving artesunate, the patient’s condition improved, and he was discharged to outpatient care 6 days later. This case study highlights the significant challenges related to nonadherence to malaria chemoprophylaxis protocols, and the shortage of artesunate in US hospitals, and suggests potential frameworks to improve prophylactic guidelines and clinical outcomes.
Keywords: Malaria; Severe malaria; Disseminated intravascular coagulation; Malaria prophylaxis; Artesunate; Blood smear; Missionary; Plasmodium falciparum
| Introduction | ▴Top |
Malaria remains a significant global health challenge, particularly in regions where it is endemic. According to the World Health Organization (WHO), there were an estimated 263 million malaria cases and 597,000 malaria deaths worldwide in 2023 [1]. The disease is particularly prevalent in sub-Saharan Africa, where 94% of malaria cases and 95% of malaria deaths occur [2]. In the United States of America (USA), malaria is relatively rare, with most cases being imported by travelers returning from endemic areas. In 2019, there were 2,048 confirmed malaria cases reported to the Centers for Disease Control and Prevention (CDC), with a significant reduction in 2020 due to decreased travel during the coronavirus disease 2019 (COVID-19) pandemic [3].
While primarily affecting regions in Africa, Southeast Asia, and South America, malaria poses a persistent threat to travelers from non-endemic areas, particularly missionaries, military personnel, and aid workers who may have stayed in endemic regions. For example, in 2023, 39 members of the US Armed Forces were diagnosed with malaria, demonstrating the ongoing risk for these individuals [4]. Nonadherence to prevention strategies among US travelers on stays in endemic regions, often due to premature cessation, missed doses or side effects, can lead to increased risk [5]. In 2018, 75% of US travelers diagnosed with malaria had not taken any prophylactic medication, emphasizing the need for improved awareness and adherence to preventive strategies [6].
One critical factor in malaria-related mortality is the timely administration of intravenous (IV) artesunate, the first-line treatment for severe malaria in the USA. Failure to promptly administer this life-saving medication can significantly increase morbidity and mortality [7]. However, timely intervention is currently complicated by the limited availability of IV artesunate in smaller hospitals [8].
This case report details the challenges faced by a missionary who traveled to Nigeria, a region where malaria is endemic, and later developed severe malaria with disseminated intravascular coagulation (DIC). The patient did not take prophylactic measures and experienced a delayed diagnosis, as his condition was initially misdiagnosed as sepsis. It was only after the patient developed severe thrombocytopenia and deterioration in his health that the medical team reconsidered the diagnosis, ultimately confirming malaria through a blood smear. The situation was further complicated by a shortage of in-house IV artesunate, which necessitated emergency transport of the medication from a tertiary care center to our facility. This study underscores the critical importance of: 1) promoting a culture of prophylactic treatment for individuals traveling to malaria-endemic areas; 2) routine screening for malaria in patients with recent travel to such regions; 3) enhancing physician awareness and training concerning malaria symptoms, diagnosis, and treatment protocols; and 4) ensuring that life-saving medications, like artesunate, are available in smaller hospitals to prevent delays in critical treatment.
| Case Report | ▴Top |
A 47-year-old male American missionary presented to the emergency department with fever, cough, and weakness for the past 2 weeks. It was of note that he had recently traveled to Nigeria for 2 months without chemoprophylaxis for malaria. When he returned to the USA 2 weeks ago, he developed fever, chills, myalgia, headache, and hematuria.
Upon admission (i.e., day 1), his complete blood count demonstrated thrombocytopenia (92,000 platelets/µL) with mild leukopenia (3,200 cells/µL). His comprehensive metabolic panel was significant for elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT) of 88 and 101 U/L, respectively. Mild hyperbilirubinemia of 1.1 mg/dL, hyponatremia of 133 mEq/L, and serum glucose of 117 mg/dL were additional abnormal findings (Table 1 [9]). Urinalysis was positive for blood and revealed a red blood cell (RBC) count of 51 RBCs/high-power field (HPF).
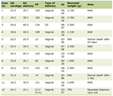 Click to view | Table 1. Comparison of Significant Laboratory Values During Hospital Course |
On day 2 of admission, malaria was seen on a peripheral blood smear (Fig. 1). Upon further review of the smear, the microscopic description showed small ringed trophozoites, some with double chromatin dots. Scattered cells were notable for the presence of dual trophozoites. No distinct gametocytes were appreciated. The leukocytes were comprised largely of granulocytes, and bands were not uncommon. Lymphocytes, while morphologically normal, were decreased in number. Occasional monocytes were noted. One thousand RBCs were counted, and 0.6% contained material parasites, equaling a parasite count of 28 × 103 parasites/µL.
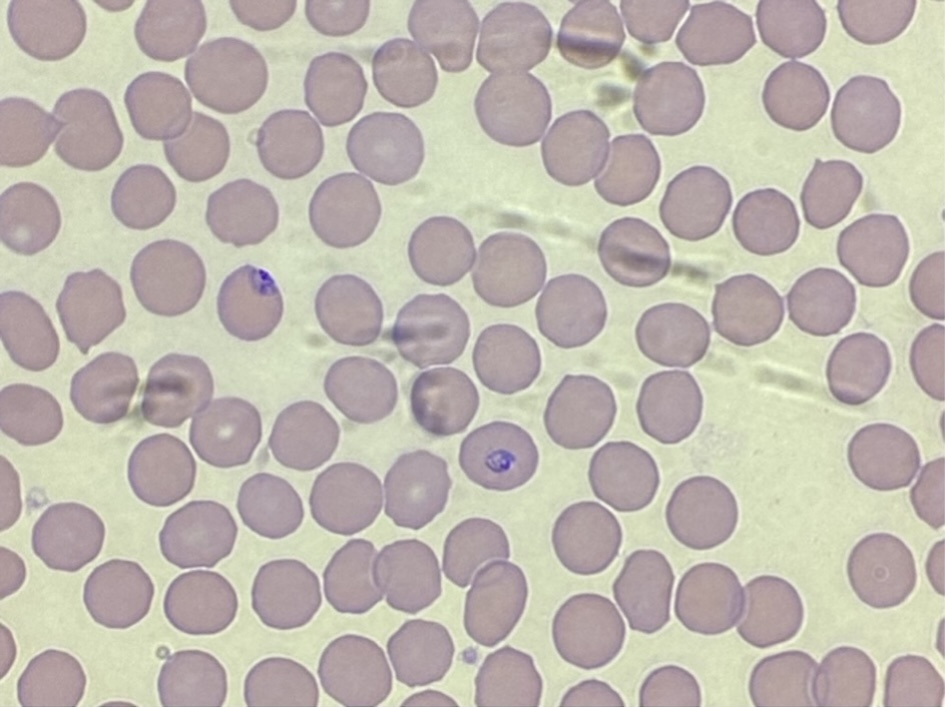 Click for large image | Figure 1. Peripheral blood smear demonstrating red blood cells infected with malaria parasites (0.6% of erythrocytes, or 28 × 103 parasites/µL). Additional hematologic findings include moderate lymphocytopenia, consistent with a diagnosis of malaria. |
As the patient had recently traveled to Nigeria, there was suspicion that the patient was infected with a resistant strain of Plasmodium falciparum. The hospital pharmacy only carried the antimalarial medications hydroxychloroquine, primaquine, and quinidine. Therefore, the patient was immediately started on quinidine 600 mg every 8 h and doxycycline 100 mg every 12 h (doxycycline shifted to one dose of clindamycin 800 mg, then 400 mg every 8 h).
On the evening of day 2, the patient became tachypneic, and the measured lactate was 3.1 mmol/L with fever. Platelets had dropped precipitously to 41,000 platelets/µL, an indication of progression to severe malaria. The process for ordering artesunate was initiated in anticipation of the development of severe malaria, but neither the pharmacy nor other local hospitals carried artesunate. The CDC was therefore contacted, and information was forwarded to the pharmacy. The patient was subsequently transferred to the intensive care unit (ICU) and was continued on quinidine and clindamycin while waiting for IV artesunate.
On day 3 of admission, the patient’s thrombocytes had declined to 25,000 platelets/µL, with a target to transfuse platelets at 20,000. Liver enzymes continued to increase throughout his stay (i.e., AST and ALT levels of 139 and 125 U/L, respectively), and his hyperbilirubinemia worsened to 2.7 mg/dL. The patient also developed hypokalemia (3.4 mEq/L) (Table 1 [9]). Diffuse petechiae were observed on his bilateral lower extremities, and coagulation studies demonstrated great concern for DIC (Table 2 [10]). As the patient had met the criteria for DIC, his infection was now classified as severe malaria. On the same day, the hospital received IV artesunate, and prompt administration was initiated. Three doses of artesunate were administered across the first 24 h at 12-h intervals, followed by one dose of artesunate each day for 2 days.
 Click to view | Table 2. Coagulation Profile and ISTH DIC Scoring |
By day 5, the patient’s thrombocytopenia had improved to 57,000 platelets/µL. On day 6 of admission, parasite levels were reduced to < 1% and the patient was switched to artemether, an oral version of artesunate. His thrombocytes and leukocytes continued to increase throughout his course, and by discharge on day 8, he had complete resolution of petechiae in the bilateral lower extremities with a thrombocyte count of 127,000 platelets/µL and improved total bilirubin (1.1 mg/dL). His hemoglobin (Hb) (12.4 g/dL) and hematocrit (36%) continued to decline steadily, and although AST had improved to 86 U/L, his ALT remained elevated at 170 U/L at discharge (Table 1 [9]). He was scheduled to follow up with his primary care physician a week later.
| Discussion | ▴Top |
Malaria is a parasitic disease caused by Plasmodium protozoan parasites and transmitted by Anopheles mosquitoes. There are five different species of Plasmodium that cause infection in humans: P. falciparum, P. malariae, P. ovale, P. vivax, and P. knowlesi [11]. The P. falciparum species is present in Western and sub-Saharan Africa and presents with the highest morbidity and mortality of the Plasmodium species. Our patient traveled to Nigeria, a country in Western Africa, and he was assumed to be infected by the P. falciparum species. Like all Plasmodium species, P. falciparum’s life cycle is divided into the sexual reproduction stage in the mosquito vector, and the asexual replication stage in humans.
Infection of the erythrocytes by merozoites marks the beginning of the “blood stage” of the asexual cycle. Innate immunity responds first via monocyte and macrophage phagocytosis of merozoite-infected erythrocytes within the splenic red pulp. Adaptive immunity then follows, responding to toxin-induced interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) by class switching of cluster of differentiation 4 (CD4)-positive lymphocytes [12, 13]. The TNF-α response to the merozoites causes the host to be anemic, and the inability of the host to keep up with the rapidly infected erythrocytes leads to massive hepatosplenomegaly [11]. The accumulated toxins released by merozoites cause systemic symptoms, such as high fever, chills, shivering, sweating, headache, abdominal and back pain, nausea, vomiting, and diarrhea. Increased splenic sequestration, immune-mediated destruction, and shortened platelet survival are potential causes of thrombocytopenia [14]. Afterwards, if an Anopheles mosquito sucks the blood of a human host with malaria, the process starts again in the mosquito vector, which may ultimately infect another human. Due to the pathophysiology of malaria, receiving antimalarial prophylaxis is highly essential.
Severe malaria is both a clinical and a laboratory diagnosis. It is most seen with the P. falciparum species. The definition of severe malaria is one or more of the following occurring in the absence of an identified alternative cause: impaired consciousness (Glasgow Coma Scale < 11), acidosis (plasma bicarbonate < 15 mM), hypoglycemia (glucose < 40 mg/dL), severe malarial anemia (Hb < 7 g/dL and hematocrit < 20%), acute kidney injury (creatinine > 3 mg/dL or blood urea > 20 mM), pulmonary edema, significant bleeding (DIC), shock or hyperparasitemia (P. falciparum > 10%) [15]. The most common and grave sign of severe P. falciparum malaria is cerebral malaria [16], specifically leading to generalized tonic-clonic seizures and eventually coma and death. Fortunately, the mortality rate of severe P. falciparum malaria has been reduced by one-third since the administration of IV artesunate [17]. The second most common sign of severe P. falciparum malaria is severe malarial anemia. Patients with Hb concentrations < 7 g/dL are considered to have severe anemia [18]. The anemia from malaria is an acute-on-chronic disease process whereby repeated or untreated malaria infections result in shortened erythrocyte survival and protracted dyserythropoeisis [19]. Metabolic acidosis is the third most common sign in those with severe P. falciparum malaria. Lactate accumulation is an important component of malaria acidosis, which is often accompanied by hypoglycemia reflecting anaerobic glycolysis and impaired hepatic gluconeogenesis [20]. Severe P. falciparum malaria is a result of extensive sequestration of erythrocytes containing mature parasitic forms of P. falciparum in the microvasculature of vital organs such as the brain, liver, kidneys, and spleen [21]. Microvascular obstruction by infected erythrocytes, consequent cellular dysfunction, and the liberation of large quantities of bioactive hemes are considered the main pathological processes in severe P. falciparum malaria [22].
Our patient was a missionary from the USA who did not receive antimalarial prophylaxis prior to his travel to Nigeria. Although missionary organizations generally provide prophylaxis before trips to endemic countries, adherence by missionaries is the primary issue. In a malaria study from 2009 and 1995, prophylaxis rates ranged from 33% to 62%, respectively [23, 24]. Among the reasons for travel, missionaries were one of the groups with the lowest proportion of chemoprophylaxis [23]. The most used prophylaxis in these studies was chloroquine, which has the advantage of a once-weekly dose, although it does not provide the immediate effect seen with doxycycline. The prophylaxis rates were highly dependent on the risk level of the area. In high-risk locations, the antimalarial prophylaxis rate was 78%. In contrast, the prophylaxis rate in low-risk areas was only 8%. It was found that 34% of the missionaries belonged to an organization that had a formulated policy on malarial chemoprophylaxis. However, only 62% of those missionaries followed the organizational policy [24].
The military’s approach to malaria prophylaxis offers valuable insights, particularly for missionary organizations. The military’s practice of directly observed therapy (DOT) is one of the most effective strategies in ensuring adherence to prophylaxis or treatment [25]. DOT entails a healthcare worker or other designated personnel watching a patient swallow each dose of a prescribed medication. In addition to ensuring appropriate prophylaxis, missionaries will also have the benefit of talking to the designated workers about issues or side effects from the medication at each encounter. DOT may either be conducted in-person or virtually over a smartphone or computer [26]. Furthermore, the use of doxycycline by military personnel, which provides rapid protection after the first dose, contrasts with chloroquine’s requirement for several weeks of pretreatment to reach protective levels. Adopting DOT and prioritizing rapid-acting prophylactics like doxycycline may significantly enhance malaria prevention efforts in missionary settings.
In cases where prophylaxis is not administered and a patient develops severe malaria, access to artesunate is crucial. IV artesunate is the first-line treatment for severe malaria, including P. falciparum infections, due to its fast-acting parasiticidal effect that leads to a dramatic reduction in parasite burden within hours of the first dose [27]. Clinical data indicate that after the first dose of IV artesunate, parasitemia decreases by approximately 50% within 4 to 5 h, by about 90% at around 15 h, and by roughly 99% after 30 h [27]. This rapid parasite clearance and prevention of complications (e.g., less hypoglycemia) is a key pharmacodynamic advantage of artesunate over older therapies like quinine [28]. Notably, the IV artesunate has been demonstrated to reduce the risk of death by about 35% versus IV quinine in severe P. falciparum malaria [29].
Our patient exhibited laboratory results indicative of DIC, which is a serious complication associated with severe malaria infection [7]. Research suggests that severe P. falciparum malaria can lead to coagulopathy and, in some instances, meet the criteria for overt DIC similar to our case [30]. The significantly reduced platelet count (25,000/µL), elevated D-dimer levels (35.2 µg/mL), and prolonged coagulation times (prothrombin time of 14.4 s, partial thromboplastin time of 39 s) aligned closely with the International Society on Thrombosis and Haemostasis (ISTH) scoring criteria, yielding a DIC score of at least 5, which meets the threshold for overt DIC (Table 2 [10]) [31]. According to CDC guidelines, a confirmed malaria infection complicated by DIC necessitates immediate initiation of IV artesunate to effectively manage severe malaria, mitigate coagulopathy, and reduce the risk of mortality [7].
Despite clear guidelines for malaria prevention, diagnosis, and treatment, the US healthcare system continues to struggle with timely diagnosis and clinical management of malaria. For example, physicians often fail to consider malaria in their differential diagnosis. As a result, these delays in diagnosis and treatment can lead to severe complications of malaria, including multiorgan failure and death [32, 33].
The limited availability of IV artesunate in smaller US hospitals poses a significant challenge to the timely treatment of malaria. Many facilities do not stock the medication due to its prohibitive cost and infrequent use; the high launch price of artesunate and small market deter many hospitals from maintaining this essential medication in their inventory [8]. The private company Amivas LLC, which received Food and Drug Administration (FDA) approval for artesunate, set the price of artesunate at over $30,000 per average treatment course, posing a significant strain on the pharmacy budget of smaller hospitals. Furthermore, only 2,000 cases of malaria are reported nationwide, only 300 of those being categorized as severe, and these facilities are hence deterred by the risk of expiration of costly artesunate vials due to a potential lack of use [34].
A few frameworks have been proposed in the literature to address the limited availability of artesunate in the USA. For example, Thomas et al proposed a hospital tier system in the approach to severe malaria that maximizes the efficiency of artesunate administration. Hospitals with in-house malaria rapid diagnostic testing (RDT), in-house microscopy, and ICUs would be considered tier-one hospitals, and they should stock artesunate abundantly as they have sufficient means to treat critically ill patients. As our hospital meets all the criteria for a tier-one hospital, it is imperative that IV artesunate is readily available in facilities like ours for severe malaria patients.
Hospitals without either an ICU or in-house microscopy but still have RDT and send-out microscopy are considered tier-two hospitals. These facilities should stock at least one dose of artesunate for prompt administration and subsequently transfer the patient to a tier-one hospital. Alternatively, tier-two hospitals may treat the malaria patient themselves instead of transferring to a tier-one hospital if microscopy results return in a timely manner for effective clinical management. In this case, the facility should stock at least one dose of artesunate in addition to oral artemether-lumefantrine. Finally, tier-three hospitals are facilities without in-house RDT, prompt microscopy, or an ICU. Patients who present to tier-three hospitals with high clinical suspicion of severe malaria should be immediately treated with an oral antimalarial agent and transferred to a tier-one or tier-two hospital for further management [8].
Conclusion
This case serves as a stark reminder of the challenges in managing severe malaria, particularly in travelers. To prevent similar outcomes, we must prioritize improved chemoprophylaxis, ensure timely access to artesunate and aggressively pursue the development of adaptable antimalarial frameworks. Further research and collaborative efforts are crucial to translate these findings into tangible improvements in patient care.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, or publication of this article.
Informed Consent
Written and verbal informed consent was obtained from the patient.
Author Contributions
All authors contributed to the drafting of the article. AL performed data analysis and interpretation and significantly contributed to the case report, discussion, and conclusion sections. SS acquired and interpreted patient data, and significantly contributed to the introduction and discussion sections. MA significantly contributed to the case report and discussion section. RY contributed to the discussion section. RD acquired patient data and supervised manuscript production and publishing. All authors have reviewed and approved the final manuscript.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
ALT: alanine aminotransferase; AST: aspartate aminotransferase; CDC: Centers for Disease Control and Prevention; CD4: cluster of differentiation 4; COVID-19: coronavirus disease 2019; DOT: directly observed therapy; DIC: disseminated intravascular coagulation; FDA: Food and Drug Administration; IFN-γ: interferon gamma; ICU: intensive care unit; ISTH: International Society on Thrombosis and Haemostasis; IV: intravenous; PTT: partial thromboplastin time; PT: prothrombin time; RDT: rapid diagnostic testing; RBC: red blood cell; TNF-α: tumor necrosis factor alpha; USA: United States of America; WBC: white blood cell; WHO: World Health Organization
| References | ▴Top |
- World malaria report 2024: addressing inequity in the global malaria response. Geneva: World Health Organization; 2024. Licence: CC BY-NC-SA 3.0 IGO. https://cdn.who.int/media/docs/default-source/malaria/world-malaria-reports/world-malaria-report-2024-spreadview.pdf?sfvrsn=3ccb3695_3.
- World Health Organization. Malaria. World Health Organization. 2024. https://www.who.int/news-room/fact-sheets/detail/malaria.
- Centers for Disease Control and Prevention. Malaria surveillance report. Centers for Disease Control and Prevention. 2024. https://www.cdc.gov/malaria/php/surveillance-report/index.html.
- Military Health System. MSMR malaria update. Health.mil. 2024. https://health.mil/News/Articles/2024/05/01/MSMR-Malaria-Update?type=Fact+Sheets.
- Mace KE, Lucchi NW, Tan KR. Malaria Surveillance - United States, 2017. MMWR Surveill Summ. 2021;70(2):1-35.
doi pubmed - Mace KE, Lucchi NW, Tan KR. Malaria Surveillance - United States, 2018. MMWR Surveill Summ. 2022;71(8):1-35.
doi pubmed - Centers for Disease Control and Prevention. Treatment of severe malaria. Centers for Disease Control and Prevention. 2024. https://www.cdc.gov/malaria/hcp/clinical-guidance/treatment-of-severe-malaria.html.
- Thomas CM, Stauffer WM, Alpern JD. Food and Drug Administration Approval of Artesunate for Severe Malaria: Enough to Achieve Best Practice? Clin Infect Dis. 2023;76(3):e864-e866.
doi pubmed - ABIM Laboratory Test Reference Ranges. American Board of Internal Medicine. 2024. https://www.abim.org/Media/bfijryql/laboratory-reference-ranges.pdf.
- Taylor FB, Jr., Toh CH, Hoots WK, Wada H, Levi M, Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327-1330.
pubmed - Buck E, Finnigan NA. Malaria. In: StatPearls. Treasure Island (FL) ineligible companies. 2025.
pubmed - Bedu-Addo G, Bates I. Causes of massive tropical splenomegaly in Ghana. Lancet. 2002;360(9331):449-454.
doi pubmed - Carlton JM. Malaria parasite evolution in a test tube. Science. 2018;359(6372):159-160.
doi pubmed - Patel U, Gandhi G, Friedman S, Niranjan S. Thrombocytopenia in malaria. J Natl Med Assoc. 2004;96(9):1212-1214.
pubmed - Severe malaria. Trop Med Int Health. 2014;19(Suppl 1):7-131.
doi pubmed - Idro R, Jenkins NE, Newton CR. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol. 2005;4(12):827-840.
doi pubmed - Artemether-Quinine Meta-analysis Study G. A meta-analysis using individual patient data of trials comparing artemether with quinine in the treatment of severe falciparum malaria. Trans R Soc Trop Med Hyg. 2001;95(6):637-650.
doi pubmed - White NJ. Anaemia and malaria. Malar J. 2018;17(1):371.
doi pubmed - Phu NH, Hien TT, Mai NT, Chau TT, Chuong LV, Loc PP, Winearls C, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Engl J Med. 2002;347(12):895-902.
doi pubmed - White NJ, Warrell DA, Chanthavanich P, Looareesuwan S, Warrell MJ, Krishna S, Williamson DH, et al. Severe hypoglycemia and hyperinsulinemia in falciparum malaria. N Engl J Med. 1983;309(2):61-66.
doi pubmed - Marchiafava E, Bignami A, Mannaberg J, Thompson JH, Felkin RW, King’s College London. Two monographs on malaria and the parasites of malarial fevers I. Marchiafava and Bignami. II. Mannaberg. London, New Sydenham Society, 1894.
doi - Leopold SJ, Watson JA, Jeeyapant A, Simpson JA, Phu NH, Hien TT, Day NPJ, et al. Investigating causal pathways in severe falciparum malaria: A pooled retrospective analysis of clinical studies. PLoS Med. 2019;16(8):e1002858.
doi pubmed - Mali S, Tan KR, Arguin PM, Division of Parasitic Diseases and Malaria, Center for Global Health, & Centers for Disease Control and Prevention. Malaria surveillance—United States, 2009. MMWR Surveill Summ. 2011;60(3):1-15.
pubmed - Burdon J. Use of malarial prophylaxis amongst a population of expatriate church workers in Northeast Zaire. J Travel Med. 1998;5(1):36-38.
doi pubmed - Whitman TJ, Coyne PE, Magill AJ, Blazes DL, Green MD, Milhous WK, Burgess TH, et al. An outbreak of Plasmodium falciparum malaria in U.S. Marines deployed to Liberia. Am J Trop Med Hyg. 2010;83(2):258-265.
doi pubmed - Centers for Disease Control and Prevention. n.d.). TB 101: Introduction to Tuberculosis. Centers for Disease Control and Prevention. https://www.cdc.gov/tb/webcourses/TB101/page16489.html.
- Kreeftmeijer-Vegter AR, van Genderen PJ, Visser LG, Bierman WF, Clerinx J, van Veldhuizen CK, de Vries PJ. Treatment outcome of intravenous artesunate in patients with severe malaria in the Netherlands and Belgium. Malar J. 2012;11:102.
doi pubmed - Dondorp A, Nosten F, Stepniewska K, Day N, White N, South East Asian Quinine Artesunate Malaria Trial g. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet. 2005;366(9487):717-725.
doi pubmed - Li Q, Weina P. Artesunate: the best drug in the treatment of severe and complicated malaria. Pharmaceuticals (Basel). 2010;3(7):2322-2332.
doi pubmed - Sailo L, Pradhan D, Nongthombam R, Bhattacharyya P. Disseminated intravascular coagulation in malaria: A case report. Niger Med J. 2014;55(2):171-172.
doi pubmed - Soundar EP, Jariwala P, Nguyen TC, Eldin KW, Teruya J. Evaluation of the International Society on Thrombosis and Haemostasis and institutional diagnostic criteria of disseminated intravascular coagulation in pediatric patients. Am J Clin Pathol. 2013;139(6):812-816.
doi pubmed - Griffith KS, Lewis LS, Mali S, Parise ME. Treatment of malaria in the United States: a systematic review. JAMA. 2007;297(20):2264-2277.
doi pubmed - Newman RD, Parise ME, Barber AM, Steketee RW. Malaria-related deaths among U.S. travelers, 1963-2001. Ann Intern Med. 2004;141(7):547-555.
doi pubmed - Rosenthal PJ, Tan KR. Expanded availability of intravenous artesunate for the treatment of severe malaria in the United States. Am J Trop Med Hyg. 2019;100(6):1295-1296.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Clinical Infection and Immunity is published by Elmer Press Inc.